All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
BRUIN trial: Phase I/II of pirtobrutinib, a novel, reversible BTK inhibitor for relapsed B-cell malignancies
When treating B cell-malignancies, covalent inhibitors correspond to the most frequently used drug class to target Bruton’s tyrosine kinase (BTK) pathway. These irreversible BTK inhibitors (BTKi) have low oral bioavailability, short half-lives, and high protein binding affinity, which results in incomplete target inhibition towards the end of the dosing interval, ultimately leading to drug resistance. Currently, up to 40% of patients treated with first-generation BTKi will discontinue the treatment due to drug resistance. Frequently, these patients harbor BTK C481 mutations.
Pirtobrutinib (also known as LOXO-305), an orally available, highly selective, reversible BTKi that can sequentially bind in a covalent and noncovalent way to its target, has been recently evaluated by Mato et al., in a first-in-human phase I/II trial in pretreated B-cell malignancies.1
Study design
- The BRUIN trial (NCT03740529) was designed as a phase I/II study.
- For the phase I part, a 3+3 dose-escalation design was used, and additional enrollment of up to 150 patients was permitted across all dose levels.
- During phase II, 323 patients were enrolled in one of six cohorts based on the type of B-cell malignancy, previous therapy exposures, and BTK mutational status. A total of 269 patients were evaluable for efficacy endpoints, and 183 patients were still on treatment by the time of this analysis.
Selection criteria
- Patients included were diagnosed with histologically confirmed B-cell malignancies, i.e., chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), Waldenström’s macroglobulinemia (WM), and other including diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL).
- Patients should have received either two failed prior standard-of-care regimens or have been intolerant to either, OR having received one prior BTK-containing regimen with a BTK inhibitor approved as first-line therapy.
- Patients with BTK C481 mutations were included.
- Enrolled patients could not have a clinically significant, uncontrolled cardiac or cardiovascular disease but could receive anticoagulants (except warfarin) and antiplatelet agents. Patients with controlled atrial fibrillation were permitted.
Dosage information
- Seven dose levels were selected: 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg, and 300 mg for a 28-day cycle of oral pirtobrutinib monotherapy.
- Treatment continued until disease progression, unacceptable toxicity, or withdrawal.
- Escalation to the next dose was permitted after the dose-limiting toxicity evaluation was complete.
Study endpoints
For phase I
- Primary endpoint: Maximum tolerated dose and the recommended phase II dose.
- Secondary endpoints: Overall response rate (ORR), pharmacokinetics, and safety.
For phase II
- Primary endpoint: ORR.
- Secondary endpoints: Best overall response, duration of response, progression-free survival, overall survival, safety and tolerability, and pharmacokinetics.
Results
Patient demographics
Patient characteristics are summarized in Table 1. Median age across the study was 68 years. Previous BTK inhibition therapy was received by 76% of all 323 patients treated with pirtobrutinib. Other previously received therapies included anti-CD20 antibody (94%), chemotherapy (87%), BCL-2 inhibitor (25%), PI3K inhibitor (16%), lenalidomide (14%), autologous stem-cell transplant (7%), allogeneic stem-cell transplant (3%), and CAR T-cell therapy (7%).
Table 1. Patient demographics*
|
BCL2, B-cell lymphoma-2; BTKi, Bruton’s tyrosine kinase inhibitor; CLL, chronic lymphocytic leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; SLL, small lymphocytic lymphoma; MCL, mantle cell lymphoma; WM, Waldenström’s macroglobulinemia. *Adapted from Mato et al.1 †Patients with diffuse large B-cell lymphoma/DLBCL (n = 26), follicular lymphoma/FL (n = 12), marginal zone lymphoma/MZL (n = 13), Richter’s transformation (n = 9), other transformation (n = 3), B-cell prolymphocytic leukemia/B-PLL (n = 2), and hairy cell leukemia/HCL (n = 1). |
|||||
|
Characteristic |
ALL (n = 323) |
CLL/SLL (n = 170) |
MCL (n = 61) |
WM (n = 26) |
Other (n = 66) |
|---|---|---|---|---|---|
|
Male, % |
66 |
64 |
77 |
69 |
61 |
|
ECOG PS, % |
|
|
|
|
27 65 6 |
|
Number of previous lines of systemic therapy (all patients) |
3 (2–5) |
3 (2–5) |
3 (2–4) |
3 (2–4) |
4 (3–5) |
|
Number of previous lines in BTK pretreated |
3 (2–5) |
4 (2–5) |
3 (2–4) |
3 (3–4) |
5 (3–7) |
|
Reasons for previous BTKi discontinuation |
|||||
|
Progressive disease, % |
71 |
67 |
77 |
67 |
79 |
|
Toxicity or other, % |
29 |
33 |
23 |
33 |
21 |
Pharmacokinetics
- Pirtobrutinib displayed linear dose-proportional exposures and low interpatient variability throughout the entire dosing range of 25 mg to 300 mg daily.
- The maximum tolerated dose level was not established as there were no incidents of dose-limiting toxicities.
- 200 mg daily was selected as the recommended phase II dose because it achieved a 96% target inhibition.
Safety
During this study, 87% of adverse events (AEs) were Grade 1 or 2. Dose interruptions were recorded in 8% of patients, dose reduction in 2%, and 1% discontinued permanently due to drug-related adverse events. Nevertheless, a longer follow-up is needed to describe the safety profile of pirtobrutinib fully.
Grade ≥3 neutropenia was observed in 10% of the overall patient population, but it was not dose-dependent. Of note, less than 1% of patients experienced atrial arrhythmias and were considered not related to pirtobrutinib. A similar safety profile was observed in patients with various tumor types and those who received at least one dose of 200 mg pirtobrutinib. Treatment-related AEs of any grade are listed in Table 2.
Table 2. TRAEs reported in the overall population (N = 323)*
|
TRAEs of any grade occurring in ≥3% of patients, % |
|
|---|---|
|
Diarrhea |
9 |
|
Contusion |
9 |
|
Fatigue |
8 |
|
Neutropenia |
6 |
|
Headache |
4 |
|
Nausea |
3 |
|
Anemia |
3 |
|
Maculopapular rash |
3 |
|
Dizziness |
3 |
|
Hyperuricemia |
3 |
|
Pruritus |
3 |
|
TRAEs of special interest, % |
|
|
Bruising |
12 |
|
Rash |
6 |
|
Arthralgia |
2 |
|
Hemorrhage |
2 |
|
Hypertension |
1 |
|
TRAEs, treatment-related adverse events. |
|
Efficacy
ORRs achieved with pirtobrutinib in the different types of malignancies are presented in Figure 1. Response distribution, median follow-up time, and patients still on treatment in every cohort at the time of data cutoff are summarised in Table 3.
Figure 1. ORR with pirtobrutinib in all efficacy-evaluable patients across all dose levels by disease*
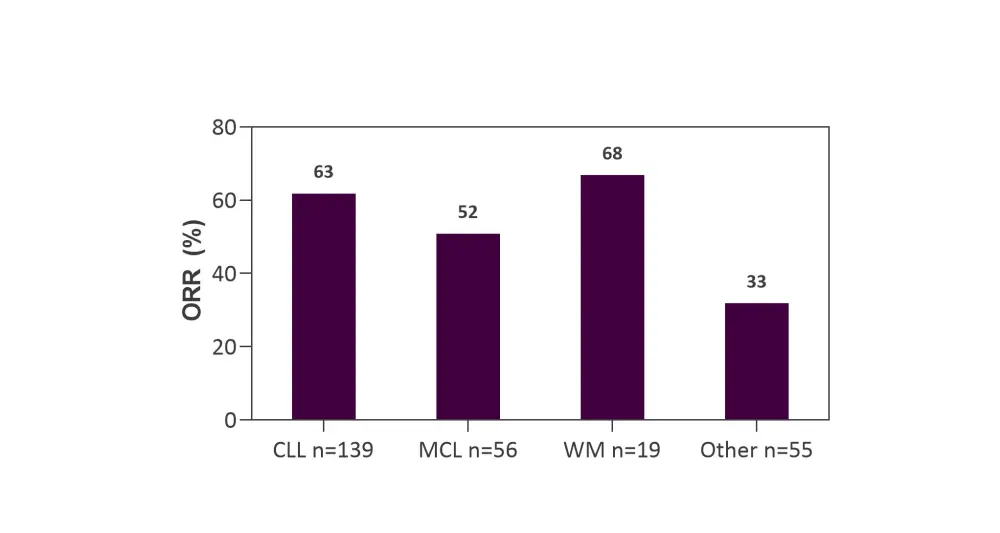
CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; ORR, overall response rate; WM, Waldenström’s macroglobulinemia.
*Adapted from Mato et al.1
In CLL/SLL
- No significant difference was observed for ORR data reported in patients with CLL/SLL who were previously exposed to a BTKi (62%, 95% CI, 53–71), even in those harboring BTK C481 mutations (71%, 95% CI, 49–87).
- A noteworthy ORR of 90% was reported in patients who received previous CAR T-cell therapy.
- Responses for CLL/SLL patients deepened over the period of 6 months (Table 3).
In MCL
- Patients with MCL achieved the same ORR (52%), irrespective of previous exposure to covalent BTKi.
- Patients who previously had received cellular therapies, such as autologous, allogeneic transplantation, or CAR-T cell therapy, also responded to pirtobrutinib.
In WM
- 69% of ORR was recorded for patients with WM who had received a previous covalent BTKi (n = 13).
Table 3. Depth of response achieved at data cutoff by patients with CLL/SLL, MCL, and WM treated with pirtobrutinib*
|
CLL, chronic lymphocytic leukemia; CR, complete response, MCL, mantle cell lymphoma; PD, progressive disease; PR, partial response; SD, stable disease; SLL, small lymphocytic lymphoma; WM, Waldenström’s macroglobulinemia |
||||||
|
B-cell malignancy |
CR, % |
PR, % |
SD, % |
PD, % |
Median follow-up, |
Patients still on treatment, |
|---|---|---|---|---|---|---|
|
CLL/SLL |
|
|
|
|
|
|
|
MCL |
25 |
27 |
18 |
21 |
6 |
57 |
|
WM |
— |
47 |
37† |
16 |
5 |
77 |
In other B-cell malignancies
Responses recorded in other malignancies were:
- 50% in FL patients (n = 8).
- 75% in Richter’s transformation (n = 8).
- 6/25 patients with DLBCL and 2/9 patients with marginal zone lymphoma (MZL) responded. 3/6 patients with DLBCL were still in remission with pirtobrutinib at a median follow-up of 4 months.
Conclusion
Pirtobrutinib shows a favorable safety profile and promising efficacy in multiple B-cell neoplasms. This novel reversible BTKi can be safely administered at full dose without the requirement of a ramp-up period. Moreover, there was no maximum tolerated dose identified.
Pirtobrutinib enables the sequential use of inhibitors that bind to the BTK receptor through covalent and noncovalent mechanisms, offering a new therapeutic alternative for patients with B-cell malignancies refractory to first- and second-generation BTKi.
With these positive preliminary results of the BRUIN study, phase III trials are ongoing to evaluate pirtobrutinib in patients previously exposed to BTKi to treat CLL/SLL (BRUIN CLL-321, NCT04666038), or MCL (BRUIN MCL-321, NCT04662255).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

