All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
CAPTIVATE 4-year update: fixed-duration ibrutinib + venetoclax in CLL and SLL
Ibrutinib, a Bruton's tyrosine kinase inhibitor (BTKi), and venetoclax, a B-cell lymphoma 2 inhibitor, have various and complementary mechanisms of action that target cell compartments and subpopulations of chronic lymphocytic leukemia (CLL) to eliminate both dividing and resting CLL cells. Ibrutinib in combination with venetoclax has been seen to provide clinically relevant benefits to patients with chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL). Three-year follow-up data from the CAPTIVATE (NCT02910583) trial were previously summarized by the Lymphoma Hub. Here, we report 4-year follow-up results presented at the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting.
Study design
CAPTIVATE is an ongoing, international, multicenter phase II study investigating ibrutinib plus venetoclax as first-line treatment for patients with CLL or SLL.1 Full details of the study design are previously summarized on the Lymphoma Hub. Eligible patients were
- aged ≥ 70 years;
- previously treated for CLL/SLL but had active CLL/SLL disease requiring treatment; and
- had an Eastern Cooperative Oncology Group score of 1–2.
The study endpoints included 4-year rate of complete response (CR) including investigator-assessed CR, overall response rate, duration of response, undetectable minimal residual disease rates (10−4 by flow cytometry), progression-free survival (PFS), overall survival (OS), and safety.
Results
Efficacy
- A total of 159 patients were included in the fixed-duration cohort.
- With 4 years of follow-up since the primary analysis reported in 2021, the best CR rate was 58% and ORR was unchanged at 96%; the median duration of treatment was 50 months.
- Four-year PFS rates were numerically lower in patients with unmutated immunoglobulin heavy chain variable or del(17p) and/or TP53 mutation, while OS rates remained consistently high. Efficacy endpoints are presented in Figure 1.
Figure 1. Efficacy outcomes*
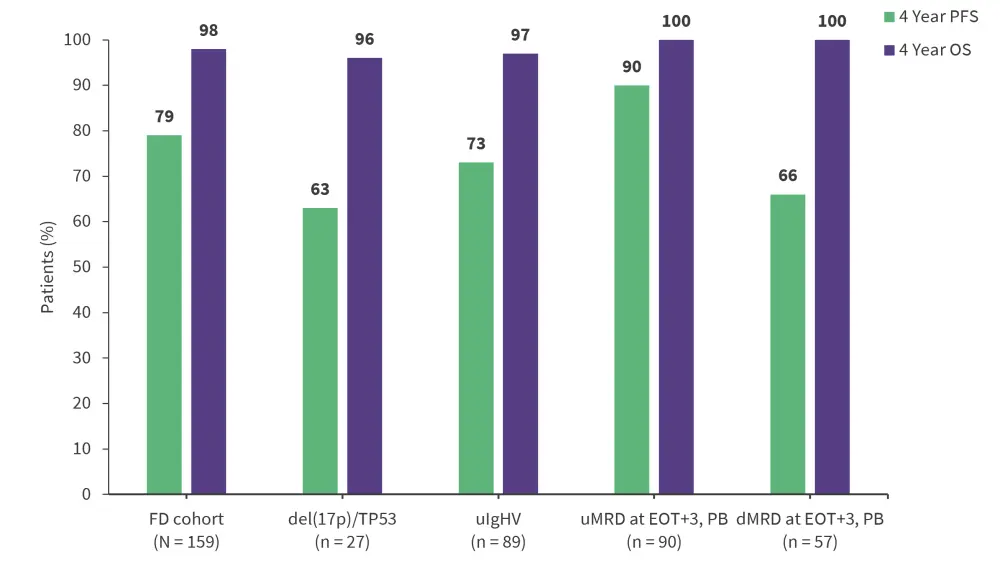
CR, complete response; CI, confidence interval; dMRD, detectable MRD, FD, fixed-duration; MRD, minimal residual disease; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; uIgHV, unmutated immunoglobulin heavy chain variable; uMRD, undetectable MRD.
*Adapted from Barr.1
- In the overall population, 21% of patients achieved undetectable minimal residual disease in peripheral blood at 36 months posttreatment; this rate was maintained from 3 months posttreatment in 23% of the evaluable population.
- One death due to COVID-19 was reported since the primary analysis.
- The median time to next treatment was not reached; landmark estimate of time to next treatment at 4 years was 84%.
Retreatment with ibrutinib
In total, 19 patients with progressive disease initiated retreatment with ibrutinib, with a median retreatment duration of 11 months; 17 of these patients responded to treatment with one achieving CR, 13 with partial response, one partial response with lymphocytosis, one stable disease, and one progressed disease. No adverse events leading to dose reduction/discontinuation were reported.
Safety
In addition to the adverse events reported in previous years, one serious adverse event, prostate cancer, occurred in this additional year of follow-up.
Conclusion
With 4 years of follow-up, fixed-duration ibrutinib plus venetoclax continued to provide clinically meaningful, deep, and durable response with manageable safety profile in patients with previously untreated CLL/SLL, including patients with high-risk disease features. Overall, the results support the use of fixed-duration ibrutinib plus venetoclax in in patients with previously untreated CLL/SLL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


