All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
CAR T-cell therapy-associated HLH-like syndrome
Hemophagocytic lymphohistiocytosis (HLH) is a severe hyperinflammatory syndrome clinically characterized by fever, hepatosplenomegaly, organ failure, neurologic toxicities, coagulopathy, cytopenias, hyperferritinemia, and/or hypertriglyceridemia as a result of underlying pathological immune activation.1
Following CAR T-cell therapy, HLH-like toxicities are emerging more widely across CAR T-cell constructs and patient populations, including in B-cell lymphomas.1 Contrary to initial reports HLH-like toxicities are increasingly recognized as not as directly associated with cytokine release syndrome (CRS) and/or its severity as initially described. These toxicities are not well-established but can be potentially fatal and life-threatening; thus, there is an urgent need to elucidate identification and management approaches.1
Herein, we summarize a recent publication in Transplantation and Cellular Therapy by Hines et al.1 on the assessment and management guidelines for HLH-like toxicities in CAR T-cell therapies, which includes their underlying biology, definition, identification, diagnostic schema, grading schema, potential treatment strategies, monitoring and supportive care strategies, as well as alternate etiologies.
Primary and secondary HLH: Underlying biology and classification
Although there is substantial similarity, HLH can be largely divided into primary and secondary categories. Primary HLH (pHLH) is an inherited genetic disorder most commonly affecting infants and young children, whilst secondary HLH (sHLH) is an acquired disease mainly seen in adults.
pHLH is most often an autosomal recessive genetic mutation affecting cytotoxic T-cell and natural killer (NK)-cell function, lymphocyte survival, or inflammasome activation. Different types of pHLH include familial HLH, Epstein-Barr Virus susceptibility disorders, lysosomal-related pigmentary disorders and X-linked lymphoproliferative diseases, and inflammasopathies.
sHLH similarly affects lymphocyte cytotoxicity, survival, or inflammasome activation and has different underlying etiologies including infections, malignancies, metabolic disorders, rheumatologic diseases (e.g., macrophage activation syndrome [MAS]), and therapy-related.
The most utilized diagnostic criteria for pHLH is HLH-2004. This is based on eight criteria including, fever, splenomegaly, cytopenias, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis along with low/absent NK-cell activity, hyperferritinemia, and high soluble interleukin (IL)-2 receptor (sIL-2R)/soluble CD25 (sCD25) levels. Other diagnostic criteria for specific patient populations and unique triggers include HScore (for adults with sHLH) and MAS/HLH criteria (for systemic juvenile idiopathic arthritis). Recent studies have reported high diagnostic reliability with the use of HLH-2004 and HScore for patients with sHLH.
CAR T-cell and therapy hyperinflammatory syndromes
Like CRS, HLH manifestations include fever, cytopenias, hyperferritinemia, coagulopathy, and hypertriglyceridemia, as well as hypotension and respiratory failure in severe cases. Additionally, overlapping cytokine (interferon [IFN]-γ, IL-6, IL-10, IL-18, sIL-2R, CXCL9) and proteomic profiles exist across both severe CRS and/or HLH.
While severe CRS coinciding with HLH-like manifestations is reported in some patients receiving CAR T-cell therapy, a different clinical entity with HLH-like manifestations after resolved/resolving CRS is observed in another subset of patients. This is now uniformly termed immune effector cell-associated HLH-like syndrome (IEC-HS).
IEC-HS
The underlying pathophysiology of IEC-HS involves CAR-T activation by tumor recognition, leading to sustained T-cell activation, proliferation, and cytokine release, subsequently followed by elevated macrophage activation and cytokine release, as depicted in Figure 1.
Figure 1. Underlying mechanism of pathologic immune activation in IEC-HS*
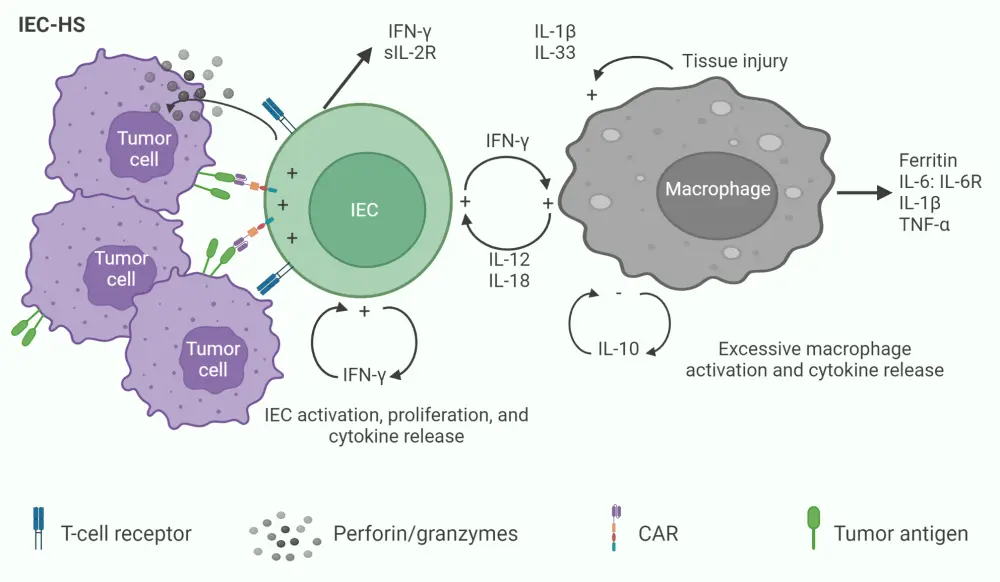
CAR, chimeric antigen receptor; IEC, immune effector cell; IEC-HC, IEC-associated HLH-like syndrome IFN-γ, interferon gamma; IL, interleukin; R, receptor; sIL-2R, high soluble interleukin 2-R; TNF-α, tumor necrosis factor α.
*Adapted from Hines, et al.1
Definition of IEC-HS
IEC-HS is formally defined as the development of a pathological and biochemical hyperinflammatory syndrome that manifests with features of MAS or HLH, is attributable to IEC-based therapy, and is associated with progression or new onset of cytopenias, hyperferritinemia, coagulopathy with hypofibrinogenemia and/or transaminitis. In addition to this, IEC-HS is often classified as a delayed manifestation occurring once CRS is resolved/resolving; though its timing of onset can vary.
Diagnostic criteria of IEC-HS
Although biochemical and pathologic similarities exist between CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), and HLH, the diagnostic criteria for IEC-HS should reflect its clinical independence from CRS. Moreover, it is critically important to distinguish between IEC-HS and severe/prolonged CRS as treatment implications differ between the two. The proposed IEC-HS diagnoses incorporate clinical and laboratory assessments based on HLH-2004, published cases, and clinical experiences; these criteria are confirmed when it is not attributable to alternative etiologies, including CRS, infection, and/or disease progression (Table 1).
Table 1. Diagnostic criteria for IEC-HS*
|
CRS, cytokine release syndrome; IEC-HS, immune effector cell associated hemophagocytic lymphohistiocytosis-like syndrome; LLN, lower limit of normal; PT, prothrombin time; PTT, partial thromboplastin time; ULN, upper limit normal. |
|
|
Manifestation |
Criteria |
|---|---|
|
Common clinical or laboratory manifestations† |
Elevated ferritin (>2 × ULN or baseline (at time of infusion)) and/or rapidly rising (per clinical assessment) |
|
Onset with resolving/resolved CRS or worsening inflammatory response after initial improvement with CRS directed therapy‡ |
|
|
Hepatic transaminase elevation§ (>5 × ULN (if baseline was normal) or >5 × baseline if baseline was abnormal) |
|
|
Hypofibrinogenemia (<150 mg/dL or <LLN)∥ |
|
|
Hemophagocytosis in bone marrow or other tissue∥ |
|
|
Cytopenias (new onset, worsening, or refractory¶) |
|
|
Other clinical or laboratory manifestations |
Lactate dehydrogenase elevations (>ULN) |
|
Other coagulation abnormalities (e.g., elevated PT/PTT) |
|
|
Direct hyperbilirubinemia |
|
|
New onset splenomegaly |
|
|
Fevers (new# or persistent)∥ |
|
|
Neurotoxicity |
|
|
Pulmonary manifestations (e.g., hypoxia, pulmonary infiltrates, pulmonary edema) |
|
|
Renal insufficiency (new onset) |
|
|
Hypertriglyceridemia (fasting level, >265 mg/dL∥) |
|
Grading schema
Considering the varied presentation of IEC-HS, with some patients presenting with fatal and/or life-threatening complications and others remaining clinically well, a grading schema to assess IEC-HS severity was proposed (Table 2).
Table 2. Grading schema for IEC-HS*†
|
IEC-HS, immune effector cell associated hemophagocytic lymphohistiocytosis-like syndrome. |
|
|
Grade |
Symptom severity and recommended intervention |
|---|---|
|
1 |
Asymptomatic or mild symptoms; requires observation and/or clinical and diagnostic evaluations; intervention not indicated |
|
2 |
Mild to moderate symptoms, with intervention indicated (e.g., immunosuppressive agents directed at IEC-HS or transfusions for asymptomatic hypofibrinogenemia) |
|
3 |
Severe or medically significant, but not immediately life threatening (e.g., coagulopathy with bleeding requiring transfusion support, or hospitalization required for new onset acute kidney injury, hypotension, or respiratory distress) |
|
4 |
Life-threatening consequences with urgent intervention indicated (e.g., life threatening bleeding or hypotension, respiratory distress requiring intubation, dialysis indicated for acute kidney injury) |
|
5 |
Death |
Treatment approaches/algorithms
There is a lack of prospective studies and paucity of data on treatment approaches in IEC-HS; thus, treatment recommendations rely on expert opinion, evidence from CRS/IEC-HS cohorts, and/or previous treatment analyses in pHLH and sHLH. Treatment analysis of pHLH are valuable to the IEC‑HS context due to the overlap in underlying pathophysiology and availability of data from large, prospective, international clinical trials (Figure 2).
Figure 2. Stepwise treatment algorithm for IEC-HS*
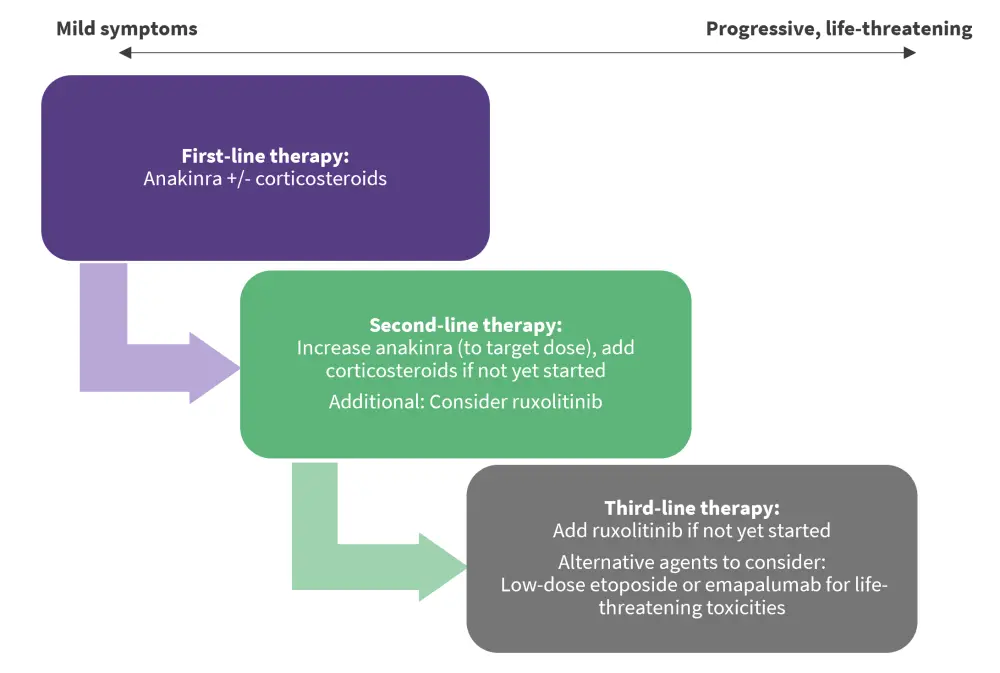
IEC-HS, immune effector cell associated hemophagocytic lymphohistiocytosis-like syndrome.
*Data from Hines, et al.1
Anakinra, an IL-1 receptor antagonist, is the recommended first-line therapy given its acceptable side effect, low toxicity, and short half-life profile. Its substantial use in the HLH context (sHLH), IL-1β upregulation in both MAS/HLH and IEC-HS contexts, and its overall expanding/emerging evidence of efficacy across CAR-T-related toxicities including CRS and ICANS, also make it a viable first-line option. Although, its optimal timing of initiation, dosing, duration, and its role remains to be elucidated.
Corticosteroids are also suitable first-line agents for IEC-HS despite the prominent side effects (metabolic derangements, hypertension, risk of infection increase, and fungal infection) and concerns about their adverse effects on CAR-T function and persistence. This is due to their short-term efficacy in CRS and ICANS, where they do not increase relapse rates. The consensus for their use is a lower dose initially, which is escalated when there is increased disease severity.
Ruxolitinib, a Janus kinase (JAK)1/2 inhibitor, is the proposed second-line treatment for IEC-HS due to its demonstrated efficacy in both front-line and salvage settings for sHLH, as well as its familiar use in the hematopoietic stem cell transplant context. It works by blocking signal transduction in the JAK/signal transducer and activator of transcription (STAT) pathway and thereby inhibiting proinflammatory cytokine activity, including IFN-γ, IL-2, and IL-6. Due to limited evidence and the potential exacerbated risk of cytopenias and infection, it is not the designated first-line approach to treating IEC-HS.
The consensus for subsequent lines of therapy is highly variable; therefore, patient-specific considerations are strongly encouraged to better guide management approaches in IEC-HS. T cell-targeted therapies (e.g., dasatinib) can be considered as a third-line option after the failure of first-line and second-line therapies in rapidly progressive/life-threatening high-grade IEC-HS. Targeted/directed switching off of the CAR T-cell therapy is the ideal method; alternatively T cell-depleting agents, such as etoposide, can be used. Etoposide is a topoisomerase II inhibitor with proven efficacy in both pHLH and sHLH settings, as seen in the largest studies (HLH-94 and HLH-2004); the preferred use of etoposide is a moderate dose of 50–100 mg/m2. Moreover, there is expanding evidence for the use of emapalumab, an IFN-γ target in CAR T-related toxicities; thus, it could be a promising option for severe cases of IEC-HS.
Supportive care strategies for IEC-HS
Beyond the treatment recommendations, several monitoring and supportive care strategies can be considered throughout the management of IEC-HS, including considerations in infection risk controls with the added use of immunosuppressive therapies (Table 3).
Table 3. Supportive care strategies in patients with IEC-HS*
|
Type of strategy |
Consideration |
|
Monitoring |
Daily monitoring of complete blood cell count with differential, coagulation parameters (PT/PTT) and fibrinogen |
|
Daily evaluation of renal and hepatic dysfunction |
|
|
Assessment for bacterial, viral reactivation or new infection, and fungal disease, in blood, urine, and sputum cultures, +/- sampling of other possible infectious sources (e.g., bronchoscopy, cerebrospinal fluid) |
|
|
Consider testing for HLH diagnostic parameters, including soluble CD25, NK-cell function, triglycerides, IFN-γ, CXCL9 ratio, CXCL10, IL-10, IL-18 |
|
|
Cytopenias |
Maintain hemoglobin >7 g/dL |
|
Platelet count >50 cells × 109/L recommended in those with active bleeding or coagulopathy |
|
|
Use of G-CSF to maintain ANC >500 cells/mm3 remains controversial during periods of active inflammation |
|
|
Consult gynecology in female patients with menorrhagia |
|
|
Coagulopathy |
Aggressive management with cryoprecipitate or fibrinogen concentrate is recommended to keep fibrinogen level >100 if no bleeding and >150 if bleeding is present |
|
If INR is >1.5, then vitamin K supplementation should be considered ; if INR is >2, then administration of fresh frozen plasma in addition to cryoprecipitate should be considered |
|
|
Cautionary use of agents for prevention of venous thromboembolism |
|
|
Consult hematology for patients with refractory or difficult-to-manage coagulopathy |
|
|
Infections |
Role for empiric management |
|
Ruling out infectious causes of sHLH |
|
|
Use of immunosuppressive therapy, prophylactic microbes in patients with IEC-HS |
|
|
Pre-emptive monitoring for infections |
|
|
ANC, absolute neutrophil count; CMV, cytomegalovirus; CXCL, chemokine (C-X-C motif) ligand; G-CSF, granulocyte-colony stimulating factor; HLH, hemophagocytic lymphohistiocytosis; HSV, herpes simplex virus; IEC-HS, immune effector cell associated hemophagocytic lymphohistiocytosis-like syndrome; IFN-γ, interferon-gamma; IL, interleukin; INR, international normalized ratio; NK-cell, natural killer-cell; PT, prothrombin time; PTT, partial thromboplastin time; sHLH, secondary HLH; VZV, varicella zoster virus. |
|
Alternative etiologies of IEC-HS
As IEC-HS can mimic other inflammatory conditions and is associated with mortality, it is crucial to distinguish these from non-IEC-HS conditions and delineate any underlying alternative etiologies to guide the best treatment choice. One factor that can be considered is additional evaluation of common IEC-HS manifestations (coagulopathy, liver injury, infections, and/or cytopenias). This can include a full assessment of coagulation and cytopenia parameters beyond the initial diagnostic work-up.
Conclusion
This expert and evidence-based framework provides a uniformed approach for identification, underlying pathophysiology, treatment strategies, supportive care, and management strategies for the newly emerged CAR T-cell therapy-associated HLH-like toxicities now known as IEC-HS. Future retrospective and prospective studies should investigate the differences between patients with severe CRS coinciding with HLH-like manifestations versus delayed IEC-HS, the difference in clinical manifestations by IEC-based therapy, risk factors for IEC-HS, and the underlying pathophysiology. This will likely inform a more comprehensive assessment and management approach and improve outcomes in patients with IEC-HS.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

