All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Chemo-free combination of lenalidomide + rituximab in elderly/frail patients with MCL and DLBCL
Mantle cell lymphoma (MCL) is acknowledged as an incurable disease with no international standard of care for elderly patients who are unfit for high-dose therapy and autologous stem cell transplant. Induction immunochemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) followed by rituximab maintenance has been found to prolong remission duration, but most patients will still eventually relapse. Recently, the chemotherapy-free combination of lenalidomide, an agent with antiangiogenic, antimalignant B-cell, and immunomodulatory activity, and rituximab has proven to be effective with a favorable safety profile in MCL. However, the benefit of adding lenalidomide to rituximab in maintenance has not been investigated so far.
Similarly, in older patients with diffuse large B-cell lymphoma (DLBCL) administering R-CHOP-like therapy is contraindicated due to comorbid conditions and a predisposition to the side effects of anthracycline containing regimens; therefore, their probability of cure is significantly reduced. Lenalidomide has been used in the setting of relapsed/refractory (R/R) DLBCL both as a monotherapy and in combination with rituximab, showing effectiveness and an acceptable safety profile. Validated tools are now also available to support clinicians in diversifying treatment regimens based on the patient’s fitness status such as the comprehensive geriatric assessment (CGA), the simplified comprehensive geriatric assessment (sCGA), and the newest elderly prognostic index (EPI).
During the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, two studies focused on the role of chemotherapy-free regimens for elderly/frail patients with aggressive non-Hodgkin lymphomas. Ribrag, et al. presented data from the MCL R2 Elderly clinical trial (EUDRACT: 2012-002542-20) which studied rituximab and lenalidomide as maintenance therapy for MCL1; while Gini, et al. explored the role of the same combination as frontline treatment in elderly frail patients with DLBCL.2 We summarize key results from both studies below.
Lenalidomide and rituximab as maintenance in MCL
Seven countries participated in this open-label, double randomized trial of the European MCL Network evaluating whether the addition of lenalidomide to standard rituximab maintenance is superior to standard rituximab maintenance for older patients with MCL.
Study design
Between November 2013 and August 2020, 620 patients were randomized for induction between 8 cycles of 3-weekly R-CHOP or 6 cycles of alternating 3-weekly R-CHOP and 4-weekly R-HAD (rituximab, cytarabine, dexamethasone); 495 of those were randomized for a second time and included in the maintenance phase (Figure 1). This article will focus on results from the maintenance phase of the study. The primary endpoint was progression-free survival (PFS), determined as the time from second randomization until progression or death from any cause, censored at the last tumor assessment date. Secondary endpoint included overall survival (OS) from induction randomization.
Inclusion criteria for the induction randomization were:
- MCL according to WHO classification with cyclin D1 overexpression or t(11;14)(q13;q12);
- ≥60 years of age and ineligible for autologous stem cell transplant;
- Ann Arbor stage II–IV;
- previously untreated; and
- ECOG Performance Status ≤2.
For the maintenance phase randomization patients had to achieve complete response (CR), CR unconfirmed (CRu), or partial response (PR) after induction treatment.
Exclusion criteria included:
- <6 cycles of R-CHOP21 or <4 alternating cycles of R-CHOP21/R-HAD28;
- calculated creatine clearance of <30 mL/min;
- ANC <1000 cells/mm3 (1.0 × 109/L); and
- platelet count <50000 cells/mm3 (50 × 109/L).
Figure 1. Maintenance phase study design*
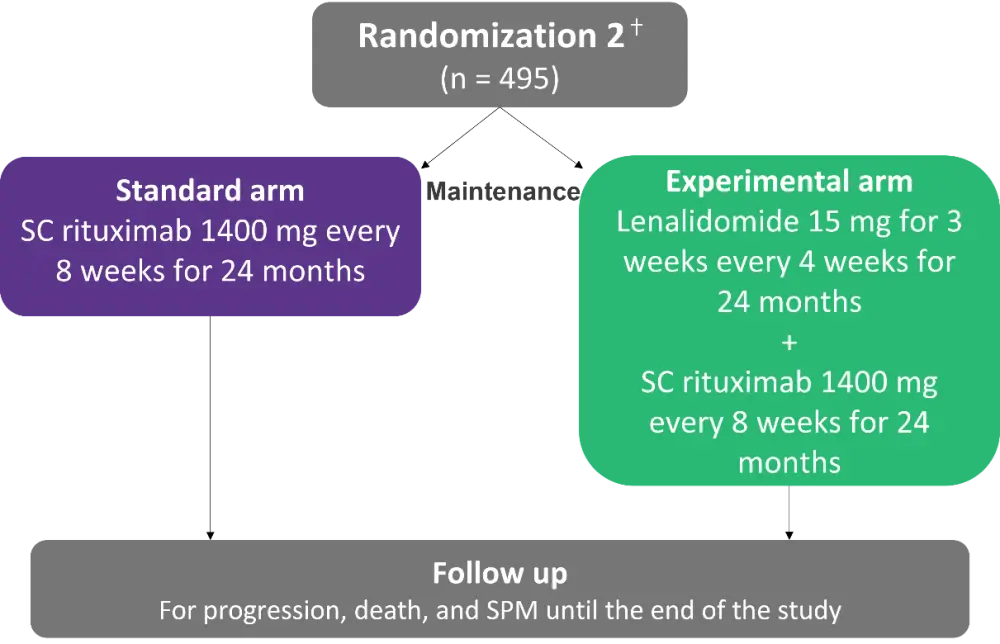
SC, subcutaneous; SPM, second primary malignancy.
*Adapted from Ribrag, et al.1
ⴕSecond randomization was stratified for induction regimen, country group, MCL International Prognostic Index (MIPI) and response (CR/CRu vs PR).
Results
Patient characteristics at maintenance are presented in Table 1 below.
Table 1. Patient characteristics at maintenance*
|
CR, complete response; CRu, complete response unconfirmed; LDH, lactate dehydrogenase; mITT, modified intention-to-treat; OR, overall response. |
|||
|
Characteristic, % (unless otherwise stated) |
Maintenance actual arm |
Maintenance mITT set (n = 447) |
|
|---|---|---|---|
|
Standard arm (n = 227) |
Experimental arm (n = 220) |
||
|
Median age, years |
71.0 |
71.0 |
71.0 |
|
Males |
70.9 |
70.0 |
70.5 |
|
Ann arbor stage III |
5.3 |
5.9 |
5.6 |
|
Ann arbor stage IV |
88.5 |
90.5 |
89.5 |
|
LDH > upper limit |
39.6 |
36.4 |
38.0 |
|
CR, CRu |
49.1 |
42.9 |
46.0 |
|
OR |
99.6 |
99.5 |
99.5 |
- After a median follow-up of 2.1 years from maintenance randomization and with 182 observed PFS events, patients receiving lenalidomide plus rituximab for maintenance had a significantly prolonged PFS in comparison to patients in the rituximab monotherapy arm (response rate [RR], 0.579; 95% confidence interval [CI], 0.429–0.781; p = 0.003).
- OS was not significantly different between the two arms, with 2-year OS of 87.3% in the experimental arm (95% CI, 81.6–91.4) and 85.8% (95% CI, 79.8–90.0) in the standard arm.
- Adverse events were more pronounced with lenalidomide plus rituximab (Table 2 and Table 3).
- In 46% of the patients in the experimental arm, the dose of lenalidomide had to be reduced at least once.
Table 2. Maintenance phase safety outcomes*
|
SPM, second primary malignancy. |
||
|
Adverse event, n |
Standard arm (n = 250) |
Experimental arm (n = 238) |
|---|---|---|
|
Blood and lymphatic system disorders |
68 |
140 |
|
Neutropenia > Grade 2 |
47 |
119 |
|
Anemia > Grade 2 |
1 |
7 |
|
Infections and infestations |
6 |
26 |
|
SPM (skin cancer) |
26 |
32 |
Table 3. Deaths during maintenance therapy*
|
*Data from Ribrag, et al.1 |
||
|
Causes of death, n |
Standard arm (n = 250) |
Experimental arm (n = 238) |
|---|---|---|
|
Lymphoma-related |
31 |
29 |
|
Toxicity of study treatment |
0 |
1 |
|
Other |
3 |
1 |
|
Total |
47 |
43 |
Conclusion
In conclusion, the addition of lenalidomide in maintenance significantly improved PFS compared with rituximab alone for patients with MCL. However, there was no difference in OS between the two arms of the study. More hematologic events and one toxic death were noted in the lenalidomide and rituximab group.
Lenalidomide and rituximab as front-line in DLBCL
This was a prospective, multicenter, single arm, phase II trial (NCT02955823) of the Fondazione Italiana Linfomi (FIL) that aimed to examine lenalidomide and rituximab as front-line chemo-free therapy for elderly frail patients with DLBCL.
Study design
From August 2018 to June 2021 68 patients were screened in 18 FIL centers, 65 patients were eventually treated in the study (Figure 2). The primary endpoint was overall response rate. Secondary endpoints included:
- complete response rate
- OS
- PFS
- event-free survival (EFS)
- safety monitoring
Inclusion criteria were:
- Ability to provide informed consent.
- Frail patients according to the CGA, newly diagnosed with DLBCL.
- Hemoglobin >10 g/d, white blood cell count >2500/mmc with PMN >1000/mmc, and platelet count >75000/mmc.
- Patients infected with hepatitis C virus and Hepatitis B cAb positive could be included but should undergo prophylaxis with lamivudine or tenofovir depending on HBV-DNA.
Exclusion criteria:
- lack of informed consent
- positive for HIV
- absence of caregivers in non-autonomous patients
Figure 2. Study design*
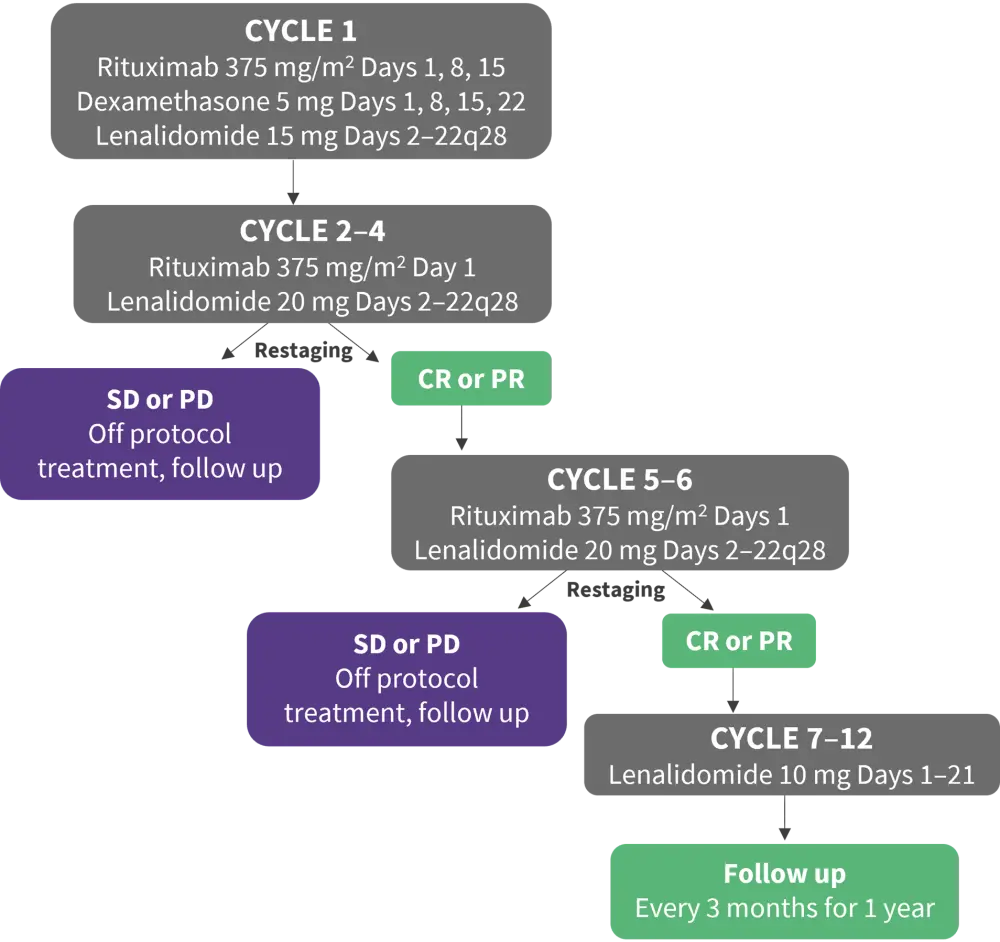
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
*Adapted from Gini, et al.2
Results
Patient characteristics at baseline are presented in Table 4.
Table 4. Baseline patient characteristics*
|
CGA, comprehensive geriatric assessment; EPI; elderly prognostic index; IPI, international prognostic index; LDH, lactate dehydrogenase; sCGA, simplified comprehensive geriatric assessment. |
|
|
Characteristic, % |
Population (N = 65) |
|---|---|
|
Age |
|
|
70–79 |
18 |
|
80–84 |
46 |
|
85+ |
35 |
|
Male |
46 |
|
Symptoms |
32 |
|
Stage III–IV |
72 |
|
ECOG Performance Status >1 |
23 |
|
LDH > UNL |
52 |
|
ENS >1 |
22 |
|
IPI 3/5 |
56 |
|
Hemoglobin <12 g/dL |
44 |
|
CGA frail |
100 |
|
sCGA |
|
|
Unfit |
28 |
|
Frail |
72 |
|
EPI |
|
|
Intermediate |
36 |
|
High |
64 |
After restaging at the end of the 4th cycle, 39 patients (60%) achieved CR or PR. At the end of induction 33 patients (80%) achieved CR or PR again and started maintenance therapy (one patient was excluded due to the physician’s decision).
- The median number of rituximab and lenalidomide cycles were 6 (1–6).
- The overall response rate was 50.7% for 33 patients instead of the required 34, so the primary efficacy endpoint was not met (Table 5).
Table 5. Response at end of induction treatment*
|
CI, confidence interval; CR, complete response; ORR, overall response rate; PD; progressive disease; PR, partial response; SD, stable disease. |
|
|
Response, % (95% CI) |
|
|---|---|
|
CR |
27.7 (17.3–40.2) |
|
PR |
23.1 (13.5–35.2) |
|
ORR |
50.7 (38.1–63.4) |
|
SD/PD |
18.5 (9.9–30.0) |
|
Early withdrawal |
18.5 (9.9–30.0) |
|
Death |
12.3 |
- Hematological adverse events (AEs) > Grade 2 were neutropenia (46.90%), thrombocytopenia (9.38%), and anemia (3.30%).
- Notable non-hematological AEs > Grade 2 included skin (10.9%), general (10.9%), respiratory (9.38%), cardiac (7.81%), infections (6.25%), central nervous system (6.25%), and gastrointestinal (6.25%).
- Overall, 48 serious AEs were noted in 37 patients including infections and infestations (9.4%), respiratory/thoracic (9.4%), and cardiac disorders (4.7%). The rate of Grade III–IV AEs was higher than the highest allowed limit of 28.
- Two patients developed SARS-COV-2 infection, and both died, one after the second cycle and one after the end of induction.
- Two-year PFS was 38% (95% CI, 25–51), with a median PFS of 14 months (95% CI, 7–NA).
- Two-year duration of remission in patients responding was 60% (95% CI, 35–78).
- Two-year OS was 45% (95% CI, 30─60), the median OS was 22 months (95% CI 15 ─ NA)
- The principal cause of death was progression of disease (Table 6).
Table 6. Causes of death*
|
*Data from Gini, et al.2 |
|
|
Cause of death, n |
|
|---|---|
|
Progression |
17 |
|
Infection |
2 |
|
Bowel infarction |
1 |
|
Pulmonary embolism |
1 |
|
Visceral arteria ischemia |
1 |
|
Heart failure |
1 |
|
Cachexia |
1 |
|
Dehydration |
1 |
|
Pneumonia |
1 |
|
Unknown |
3 |
|
Total |
29 |
Conclusion
Although the chemotherapy-free regimen of lenalidomide and rituximab did not meet the primary endpoints of the study, activity was demonstrated in a significant proportion of elderly/frail patients with DLBCL, warranting further exploration of a chemo-free approach for these patients. The study also established that CGA is a useful tool to design treatment in elderly patients with DLBCL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

