All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Circulating microRNAs in cerebrospinal fluid and plasma: a new diagnostic modality in lymphoma treatment
Do you know... Monitoring levels of oncogenic microRNAs (oncomiRs) has the potential to detect secondary CNS involvement, reflect therapy efficacy, and predict CNS relapse in aggressive B-NHL lymphomas. According to a recent analysis of 162 patients with aggressive B-NHL, how far in advance might trends of circulating oncomiR levels in plasma and cerebrospinal fluid (CSF) be used to predict complete disease remission?
Lymphoma involving the central nervous system (CNS) and CNS relapse present diagnostic and predictive challenges. Current diagnostic methods have low sensitivity and/or specificity; therefore, better tools are required for adequate early detection, response evaluation, and accurate CNS relapse prediction. MicroRNAs are small post-transcriptional gene regulators, with a high degree of stability, that are detectable in extracellular bodily fluids. Krsmanovic, et al., recently published an article in Cancers (Basel) examining the diagnostic and predictive potential of circulating oncogenic microRNAs (oncomiRs) in cerebrospinal fluid (CSF) and plasma for the detection of secondary CNS involvement in aggressive B-cell non-Hodgkin lymphomas (B-NHL), and for detection and prediction of CNS relapse. The findings indicate that these biomarkers may provide a precise tool for the early detection of secondary CNS lymphoma, evaluation of treatment efficacy, and prediction and early detection of CNS relapse. Below, we summarize the results.1
Lymphoma with secondary CNS involvement is a highly aggressive malignancy, with a poor prognosis and high mortality rate. Median overall survival (OS) is approximately 4–5 months, with a 2-year OS rate of 10–20%. CNS involvement can be characterized as primary or secondary (SCNSL), with the latter occurring in 5–25% of all systemic non-Hodgkin lymphomas. Current diagnostic modalities for the detection of CNS lymphoma involvement include magnetic resonance imaging or computed tomography followed by brain biopsy, flow cytometry, cytology, and biochemical examinations of the CSF. These methods confer a low degree of sensitivity and/or specificity for the detection of CNS involvement; therefore, new diagnostic tools are needed.
MicroRNAs are small, non-coding RNAs (19–23 nt), that downregulate gene expression via translational repression and/or mRNA degradation. They are often dysregulated in hematologic malignancies, in which they show tumor type-specific expression and can affect tumor biogenesis. Many microRNAs have also exhibited pronounced stability in bodily fluids and upregulation in the presence of systemic lymphomas, making them ideal candidates as tumor biomarkers and demonstrating their diagnostic potential in the context of primary CNS lymphoma. However, there is no clear data on the association of these molecules with secondary CNS lymphoma.
Objectives
- The primary objective was to evaluate the diagnostic and predictive potential of oncogenic microRNAs in CSF and plasma for the detection of secondary CNS involvement in aggressive B-NHL.
- A secondary objective was to determine their utility for the detection and prediction of CNS relapse in these same disease subtypes.
Study design
- Paired samples of CSF, plasma and serum were collected from 162 patients with B-NHL diagnosed with diffuse large B-cell lymphoma (DLBCL; n = 97), mantle cell lymphoma (MCL; n = 34), Burkitt lymphoma (BL; n = 18) or B-NHL-not otherwise specified (NOS; n = 13; Figure 1).
- The patient cohort also included 22 age- and sex-matched non-lymphoma patients to act as a control (neurological disorders, n = 12; no known disease/disorder, n = 8; encephalitis n = 4; FPH, n = 3, multiple sclerosis, n = 3; Sjogren syndrome, n = 2, vascular infarction, n = 2).
- In total, there were 32 CNS relapses. This was further subdivided into those with CNS relapse at time of diagnosis, termed SCNSL-dg (n = 3), and those who had samples taken when CNS relapse was detected, termed current relapses (n = 20). In addition, there were a number of subsequent relapses that occurred during the follow-up period (n = 11).
- Five microRNAs (oncomiRs: miR-21, miR-19a, miR-20a, miR-92a, and miR-155) were selected, from a pool of 20 candidates, to be evaluated for their ability to detect secondary CNS lymphoma involvement.
- Individual oncomiR abundance was evaluated as a singular variable by the creation of a logistic regression model, combining individual oncomiR abundance into a single classifier called the oncomiR index.
Figure 1. Breakdown of samples by lymphoma subtype and systemic vs SCNSL involvement*
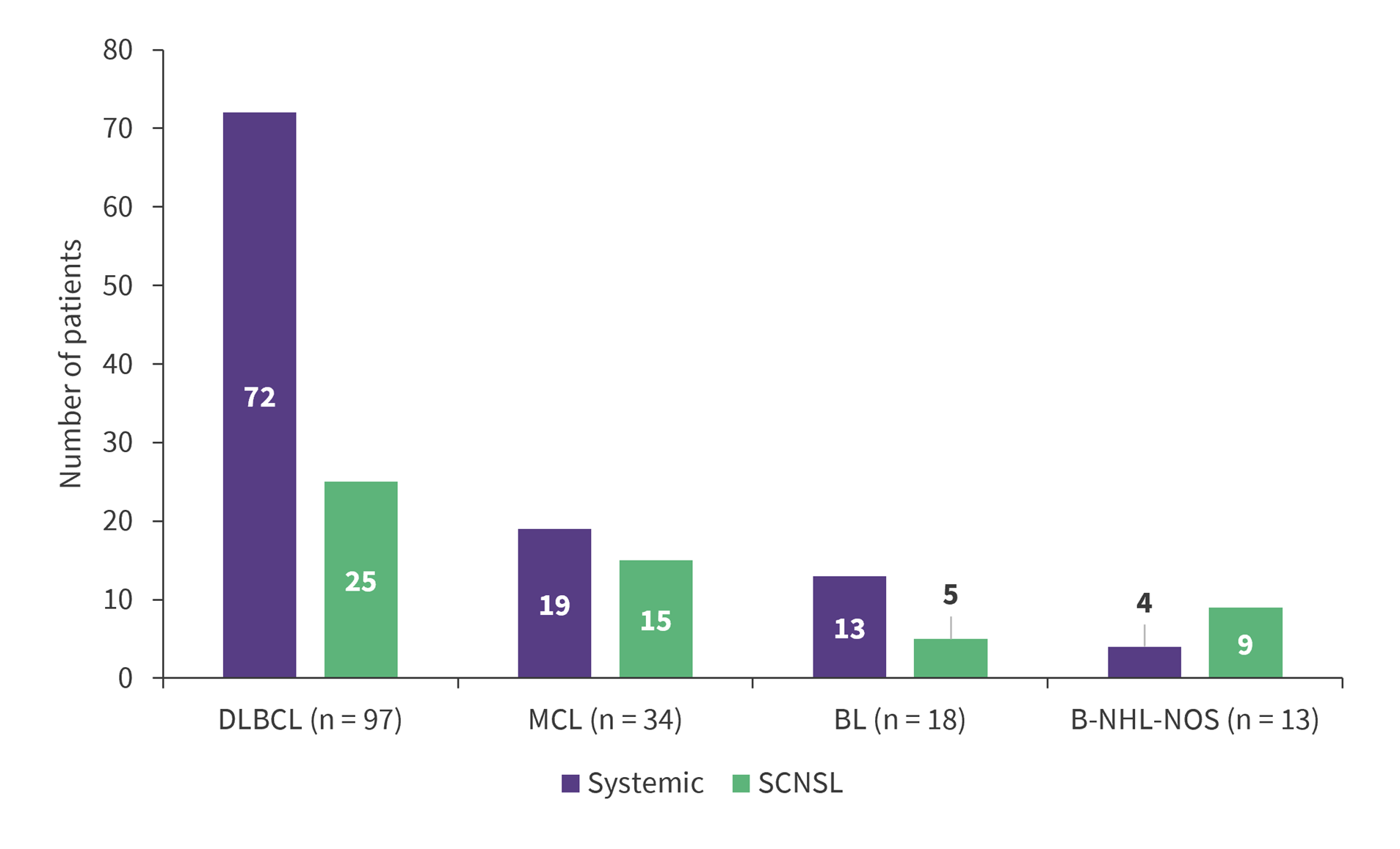
BL, Burkitt lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; NOS, not otherwise specified; SCNSL, secondary central nervous system lymphoma.
*Adapted from Krsmanovic, et al.1
Can OncomiRs be used to detect CNS involvement?
When CSF samples taken from patients with CNS-involving lymphomas were compared with those taken from the control group, all SCNSL subtypes had significantly increased levels of all five selected oncomiRs (miR-19a, miR-20a, miR-21, miR-92a, and miR-155). In addition, these CNS-involving lymphoma samples were compared with their corresponding systemic disease samples; for DLBCL samples, all tested oncomiRs were significantly increased in SCNSL compared to systemic lymphoma. Furthermore, samples taken from patients with MCL, BL, and B-NHL-NOS also showed elevated microRNA levels in comparison to systemic lymphoma, albeit to a lesser extent.
Utilization of the oncomiR index significantly improved the separation of CNS lymphoma compared to systemic lymphoma for all lymphoma subtypes and provided a high degree of sensitivity/specificity (DLBCL, 91/90%; MCL, 88/93%; BL, 100/100%; B-NHL-NOS, 100/89%). This suggests that the oncomiR index could provide a useful means of differentiating CNS lymphoma from systemic lymphoma. CNS lymphoma was also stratified in terms of parenchymal and leptomeningeal involvement. Both localizations showed a comparable increase of oncomiR indices, indicating that circulating CSF oncomiRs, in contrast to currently used methods, are able to detect parenchymal involvement.
Are CNS involvement and CNS relapse detectable in plasma?
OncomiR levels in plasma were also determined and compared in the same manner as the CSF samples. Similar trends were seen here, with an increase of all plasma oncomiRs in all CNS lymphoma subtypes observed when compared with controls as in CSF. Differentiation of CNS lymphoma from the control group was near or equal to 100% (AUC = 1) in some instances, as reflected by a high degree of sensitivity and specificity. However, the oncomiR increase and separation of CNS from systemic lymphoma in plasma was less than that seen in CSF samples.
In DLBCL and MCL, a significant increase in plasma oncomiR indices and individual oncomiR abundance was observed in cases of CNS relapse compared with patients with CNS involvement at time of diagnosis. This phenomenon was not seen in CSF, indicating that the spread of disease from systemic disease to the CNS could be characterized by an increase in plasma oncomiRs.
Can oncomiR levels show therapy efficacy and does increase precede CNS relapse?
To determine the subtleties of oncomiRs in CSF and plasma during therapy, samples were collected at multiple timepoints during therapy. Analysis revealed that in the subset of therapy responders, remission was preceded by a gradual decrease in oncomiR levels in both plasma and CSF samples. This reduction was able to predict complete remission up to 10 months in advance. In comparison, refractory lymphoma exhibited a gradual increase of oncomiRs in CSF and plasma (not all subtypes) during treatment. Similar predictive potential could also be seen in systemic patients who developed CNS relapse.
Longitudinal analysis indicated that;
- oncomiR levels can quickly change in response to disease state and therapy;
- observable trends in oncomiR levels can predict therapy efficacy and patient outcomes as evaluated by conventional methods; and
- CNS relapse and/or progression of secondary CNS and systemic lymphoma is preceded by 1–4 months of increasing oncomiR levels in both CSF and plasma.
Can OncomiRs predict CNS Relapse in DLBCL?
It was also hypothesized that a predisposition to CNS relapse due to elevated levels of CNS-specific oncomiRs at the point of lymphoma diagnosis may exist. In order to test this, oncomiR levels in CSF and plasma of patients with systemic DLBCL and DLBCL with secondary CNS involvement at time of diagnosis were analyzed, and the subgroup of those who subsequently experienced CNS relapse was compared with those who did not. The CNS relapses of secondary CNS DLBCL had oncomiR levels among the highest compared to the non-relapsing group, in both CSF and plasma. OncomiR levels were also increased in cases of subsequent CNS relapse or progression of DLBCL. This indicates that patients with increased levels of oncomiRs at diagnosis could be more at risk of CNS relapse, in both secondary CNS and systemic lymphoma.
Subsequent statistical analysis and the use of predictive models indicated that the predictive capabilities of oncomiRs increased with approaching CNS relapse, consistent with findings from the longitudinal analysis. Furthermore, patients with a high level of oncomiRs had lower rates of OS, further illustrating the prognostic value of oncomiRs. Overall, these data taken together make a strong case for the utility of oncomiRs to predict CNS relapse and OS.
Conclusion
The authors demonstrated that the expression of circulating oncomiRs (miR-19a, miR-20a, miR-21, miR-92a, and miR-155) is similarly elevated in DLBCL, MCL, and BL. They represent an early and sensitive marker of CNS lymphoma involvement, while also allowing for the accurate differentiation of CNS-involving lymphomas from systemic-only lymphomas. The application of a longitudinal regression model that combined individual microRNAs into a single classifier provided a high degree of separation between secondary CNS lymphoma and systemic lymphoma involvement. The findings indicate that circulating CSF oncomiRs, incorporated into the oncomiR index, can serve as an indicator of CNS involvement of aggressive B-NHL, and potentially other forms of lymphoma. Statistical analysis also elucidated the threshold values of this index for detecting CNS lymphoma involvement. The established thresholds are valid for both concurrent systemic and secondary CNS involvement at the time of initial diagnosis of secondary CNS lymphoma, as well as for newly detected CNS relapses.
In summary, the results describe a set of circulating oncogenic microRNAs termed oncomiRs. When combined into the oncomiR index, they represent an accurate and sensitive tool for the detection of secondary CNS B-NHL, including DLBCL, MCL, and BL. They have an advantage over conventional diagnostic modalities in that they can detect early and residual CNS lymphoma involvement, as well as parenchymal involvement in DLBCL, while also having the ability to predict clinical responses to chosen therapies. They can also provide an accurate prediction of CNS relapse risk.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

