All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Educational theme: Mutational profile and treatment of Waldenström’s macroglobulinemia
Over the next month, the Lymphoma Hub will be focusing on a new educational theme: Advances in treating Waldenström's macroglobulinemia (WM). This article follows on from our introductory piece, which can be found here.
During the Virtual 46th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), three talks on WM were delivered by Veronique Leblond,1 Ramón García-Sanz,2 and Charalampia Kyriakou.3
How biology may affect treatment strategy in WM1
Veronique Leblond presented the opening talk by discussing whether specific mutations in WM can be used as a diagnostic, prognostic, or predictive tool. The talk focused on three genes that are known for their importance in WM: MYD88, CXCR4, and TP53.MYD88 mutations are present in > 90% of patients with WM with the most common variant being L265P (98% of cases). This mutation occurs as an early clonal event and is not exclusive to WM. A small percentage of cases retain the wild type (wt) MYD88 and these patients have a distinct phenotype when compared to those with the L256P mutation (wt vs L265P):
- Females overrepresented (43% vs 24%; p = 0.001)
- Lymphocytosis (24% vs 5%; p = 0.006)
- High lactate dehydrogenase (371 UI/L vs 265 UI/L; p = 0.002)
- Decreased bone marrow infiltration (23% vs 33%; p = 0.005)
MYD88 has the potential to be used as a diagnostic tool for patients with extramedullary disease. The presence of MYD88 was found in the cerebrospinal fluid and pleural effusions of patients with WM, even in the absence of malignant cells in the samples.
Mutations as prognostic tools
The question of whether these mutations can be used as a prognostic tool was addressed next. The presence of wt MYD88 has been shown to be an independent risk factor for progression to symptomatic WM or transformation to diffuse large B-cell lymphoma.
Patients with CXCR4 mutations tend to show a more aggressive phenotype. Out of the > 50 CXCR4 variants the S338X (WHIM) mutations are the most common (35−50% of patients). These are usually nonsense or frameshift mutations, and unlike the case with MYD88 they are a subclonal event. Patients with a CXCR4 mutation tend to have:
- Increased bone marrow infiltration and cytopenia
- Reduced tumor mass
- Increased genomic complexity and IgM
- More aggressive presentation
The value of CXCR4 as a prognostic tool is not clear. Positive results have been gained from murine studies but not from patients with WM.
The del 17p TP53 mutation is rarer, with only 8% of patients with WM presenting with it. TP53 mutations have been associated with shorter time to treatment, response to treatment, and overall survival (OS).
Mutations as a predictive tool
In two small studies, patients with wt MYD88 or CXCR4 mutations who were being treated with ibrutinib demonstrated an association with treatment resistance and a lower response rate. Interestingly, the type of CXCR4 mutation impacts the survival of patients with WM. Nonsense CXCR4 mutations lead to worst OS and progression free survival (PFS) when compared to frameshift CXCR4 mutations. Investigating the cancer cell fraction CXCR4 S338X:MYD88 L265P showed that a fraction rate of ≥ 25% (high CXCR4 S388X clonality) was associated with shorter PFS and lower rates of very good partial response (VGPR).
However, these results have been challenged by the ACE-WM-001 (NCT02180724) and ASPEN (NCT03053440) studies that used new generation Bruton’s tyrosine kinase (BTK) inhibitors acalabrutinib and zanubrutinib, respectively. Both studies showed that MYD88 mutational status had no impact on treatment outcome. Similarly, in patients who are treated with rituximab plus bendamustine or proteasome inhibitors, or anti-CD20 antibodies plus B-cell receptor inhibitors, CXCR4 mutational status does not appear to impact survival outcomes.
In patients with TP53 mutations, BTK inhibitors like ibrutinib did lead to a positive response, making it a possible treatment option for this patient subset.
There is great potential for the use of mutations as diagnostic and prognostic tools for WM. Veronique Leblond recommends a genomic driven treatment algorithm for patients with WM:
- mut MYD88, wt CXCR4:
- BR, proteasome inhibitors (PI)-R, cyclophosphamide-R or ibrutinib
- wt MYD88, wt CXCR4:
- BR, PI-R, or ibrutinib-R* Pi3k inhibitors + vincristine
- mut MYD88, mut CXCR4:
- If a fast response is not required follow option 1, otherwise use option 2
- TP53 disruption:
- BTK inhibitors* Pi3k inhibitors*
*requiring further study to confirm.
Conventional and new drugs2
Ramón García-Sanz presented a talk about the current therapeutic options in WM and future treatment options. There is a wealth of new drugs in WM, so how to choose between regimens becomes an important topic. This talk highlighted that the only patients with WM that need to be treated are patients showing symptoms, as these tend to have a much poorer OS than asymptomatic patients.
Treatment criteria for symptomatic WM:
- Recurrent fever, night sweats, weight loss, fatigue
- Hyperviscosity
- Lymphadenopathy (symptomatic or bulky with ≥ 5 cm max diameter)
- Symptomatic hepatomegaly and/or splenomegaly
- Symptomatic organomegaly and/or organ or tissue infiltration
- Peripheral neuropathy due to WM
- Symptomatic cryoglobulinemia
- Cold agglutinin anemia
- Immune hemolytic anemia and/or thrombocytopenia
- Nephropathy related to WM
- Amyloidosis related to WM
- Hemoglobin ≤ 10 g/dL
- Platelet count < 100 x 109/L
For the treatment of first-line WM, patients are stratified into four groups based on whether they are fit or unfit and their tumor burden (low or high), according to the 2019 ESMO guidelines as shown in Table 1. This can be difficult in certain cases, but there are common treatments that can be used for borderline patients. For example, rituximab/cyclophosphamide/dexamethasone (DRC) can be used in fit patients who are borderline between high and low tumor burden.
Table 1. Treatment algorithm for patients with WM depending on tumor burden and patient fitness2
|
BR, bendamustine + rituximab; DRC, rituximab + cyclophosphamide + dexamethasone; VDR, bortezomib + rituximab + dexamethasone; VR, bortezomib + rituximab; WM, Waldenström’s macroglobulinemia. |
||||
|
Patient status |
Fit |
Unfit |
||
|---|---|---|---|---|
|
Tumor burden |
Low |
High |
Low |
High |
|
Treatment |
DRC x 6 BR x 4−6 VDR x 5 VR x 6 Ibrutinib 420 mg/d |
DRC x 6 VDR x 5 Ibrutinib 420 mg/d |
Oral fludarabine x 6 Rituximab x 8 Ibrutinib 420 mg/d Chlorambucil x 12 |
Ibrutinib 420 mg/d BR x 4 |
The talk then focused on three groups of treatments:
- Chemo-immunotherapy
- BTK inhibitors
- Other drugs
Chemo-immunotherapy
Rituximab combined with:
- Cyclophosphamide (CD-R)
- Bendamustine (BR)
- Anthracyclines (CHOP-R)
- Bortezomib and dexamethasone (VD-R)
Monotherapy:
- Chlorambucil
- Oral fludarabine
When using rituximab, an IgM flare may occur during the first two months of therapy, which can lead to two major issues. Firstly, it may trigger hyperviscosity syndrome, which can be prevented by either chemotherapy and rituximab or prior plasmapheresis. Secondly and most importantly, patients may still exhibit a high IgM level 2−3 months after treatment, which may be confused with treatment resistance. Therefore, it is important to give ibrutinib-related treatments sufficient time to act (4−6 months).
Three of the most commonly used chemo-immunotherapy regimens are compared in Table 2. BR appears to be the most promising of the group leading to a greatly prolonged PFS of 69.5 months compared with 35.0 and 31.2 months for DRC and R-CHOP, respectively. However, it must be noted that BR may produce toxic side effects, such as cytopenias and infections, necessitating the reduction of therapeutic doses or premature treatment termination. Maintenance therapy with BR after BR frontline did not lead to any clinical benefit in terms of PFS or overall response rate (ORR) when compared to ceasing treatment after first line therapy.
Table 2. Comparison of DRC, R-CHOP and BR regimens for WM2
|
Regimen |
DRC |
BR |
R-CHOP |
|---|---|---|---|
|
BID, twice daily; BR, bendamustine + rituximab; DRC, rituximab + cyclophosphamide + dexamethasone; IV, intravenous; ORR, overall response rate; PFS, progression-free survival; PO, oral; R-CHOP, rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone. |
|||
|
Dosing |
Dexamethasone, 20 mg IV - Day 1 Rituximab, 375 mg/m2 IV - Day 1 Cyclophosphamide, 100 mg/m2 PO BID - Days 1−5 (total 1000 mg/m2) |
Bendamustine, 90 mg/m2 - Days 1 & 2 Rituximab, 375 mg/m2 - Day 1 or 2 |
Rituximab 375 mg/m2 on Day 1 of each cycle. Cyclophosphamide 750 mg/m2, Doxorubicin 50 mg/m2, Vincristine 1·4 mg/m2 - Day 1 Prednisone 100 mg/day for 5 days4 |
|
Frequency and duration |
Repeated every 21 days for 6 courses |
Every 4 weeks for 6 courses |
3-week cycles for 6 courses maximum |
|
ORR (%) |
83 |
96 |
94 |
|
PFS (months) |
35 |
69.5 |
28.1 |
Bortezomib is another alternative treatment, which results in comparable ORR when used with rituximab (87%) but constitutes a shorter PFS (16 months). Two studies have shown that bortezomib outcomes for WM can be improved when combining it with rituximab and dexamethasone (VDR), leading to an ORR of 96% and a PFS of 69 months.2
BTK inhibitors
Ibrutinib is a first-in-class BTK inhibitor that has shown very promising results when treating patients with relapsed/refractory (R/R) WM. This was apparent in the iNNOVATE phase III trial (NCT02165397), which reported a 30-month PFS of 82% and 30-month OS of 94%, when using ibrutinib plus rituximab.5 This benefit against the placebo plus rituximab arm was also sustained in patients with poor molecular prognostic features, like MYD88 and/or CXCR4 mutations. Second generation BTK inhibitors, such as acalabrutinib, have also entered the WM treatment setting and have been used as first- and second-line therapy with similar results. In a recent phase II trial (NCT02180724), treatment-naïve patients (n = 14) who received acalabrutinib, achieved:
- ORR of 93%
- 24-month PFS of 90%
In the R/R group of patients (n = 92), acalabrutinib treatment led to:
- ORR of 94%
- 24-month PFS of 82%
In terms of toxicity, very few serious adverse events (AEs) were witnessed with acalabrutinib. The most common were bleeding (Grade 3−4 < 3%), atrial fibrillation (5%), and hypertension (5%).
Zanubrutinib is another second generation BTK inhibitor with higher specificity, thus it is expected to cause fewer off-target side effects. In the phase III ASPEN trial, zanubrutinib led to fewer cases of discontinuation due to AEs (4.0%) when compared to ibrutinib (14.3%). Tirabrutinib has also been tested in a small trial of 27 patients with WM (18 treatment naïve, nine R/R) and has shown promising preliminary results with an ORR of 94.4% in treatment naïve and 100% in R/R patients.
Other drugs
While there are many drugs that show promise for the treatment of patients WM, venetoclax was the primary focus of the speaker. In a phase II trial, venetoclax showed an ORR of 87% in patients who had received at least one prior line of therapy. Neutropenia was the most common AE recorded.
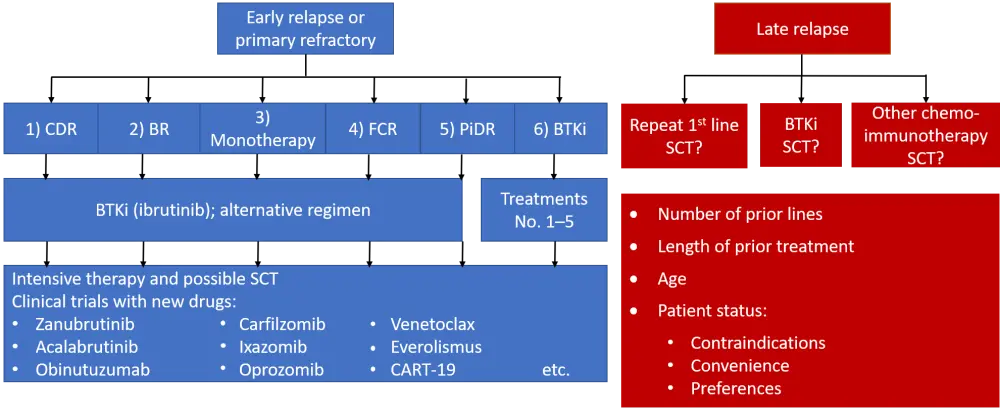
The talk concluded with the treatment algorithm shown in Figure 1, which summarizes the options for second-line treatment of patients with WM.
Figure 1. Treatment algorithm for patients with WM2
Hematopoietic stem cell transplantation in WM3
Charalampia Kyriakou presented the last talk of the session and highlighted that with the great advances in the field and the numerous available treatments for patients with WM, physicians can now choose the best option for their patients. Therapies can be tailored to individuals depending on their specific needs, molecular status, or acquired treatment resistance. Nevertheless, sequencing of therapies must be optimized in order to achieve the best patient outcomes, given that there is now a choice between continuous therapy and fixed-term treatment.
It is important to question the role of stem cell transplantation (SCT) in the treatment of WM. While there is limited data on the role of SCT in patients with R/R WM who have been treated with multiple lines of therapy, a few small studies have indicated that these patients can be salvaged by intensive treatment and SCT.
The use of both autologous (auto)- and allogeneic (allo)-SCT has increased since its introduction in the 1990s, though auto-SCT tends to be used more, Nevertheless, with the introduction of new therapies the last couple of years there has been a drop in both types of SCT.
Auto-SCT
In an EBMT analysis of 158 patients who underwent auto-SCT following treatment with multiple lines of therapy, > 75% of patients achieved a VGPR or complete response (CR). Also, OS and PFS for patients who had auto-SCT after previously achieving VGPR or partial response (PR), were significantly improved compared to patients with less than PR prior to auto-SCT. Patients with chemosensitive disease exhibited better PFS and OS after auto-SCT than chemorefractory patients (p < 0.001). In addition, this study demonstrated a late non-relapse mortality rate of 5.6% at 5 years after auto-SCT, which was due to the development of secondary malignancies. It was pointed out that alkylating agents were favored at this time.
Updated results from the EBMT in 615 patients following auto-SCT confirmed these results with patients achieving an ORR of 89%. The relapse rate was 35.1% and the incidence of secondary malignancies 7.2%.
In patients undergoing a second auto-SCT (n = 81), PR or VGPR was achieved by 55% while the incidence of secondary malignancies in this group was reported at 2.6%.
Again, patients who had previously responded to treatment showed improved outcomes after auto-SCT than patients with chemoresistant disease.
Allo-SCT
Allo-SCT is normally used as the last resort for patients who have exhausted all other treatment options. The trend from a small number of studies indicates that salvage therapy might be possible with allo-SCT, although survival outcomes are negatively impacted by the increased number of AEs associated with this therapy.
In a group of 25 patients with high risk WM and heavy pre-treatment (44% with chemorefractory disease), OS and PFS after allo-SCT plateaued at 67% after 3 years. Similar outcomes were also reported by the EBMT Working Group. In this study, chronic graft-versus-host disease (GvHD) development occurred in 21 patients, and these patients had a significantly lower risk of relapse suggesting the graft-versus-WM effect was occurring too (p = 0.03). In further support to this effect, rescue from GvHD was possible with donor lymphocyte infusions with 80% of patients achieving PR or CR.
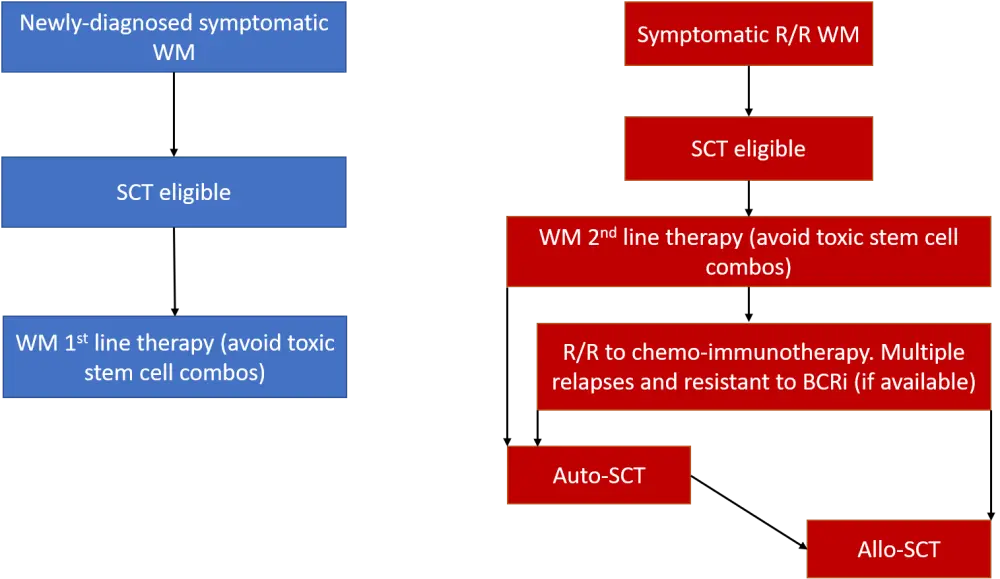
A project was set up by the EBMT Working Lymphoma Party to reach a consensus on the indications for SCT in WM. This project agreed that SCT was not appropriate for patients undergoing first-line therapy but is appropriate following second or subsequent relapses in patients with WM. The consensus statements reached are summarized in Figure 2.
Figure 2. SCT treatment algorithm for WM3
The Lymphoma Hub will be covering all the latest updates on the treatment and management of WM over the next month, so stay tuned for more!
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

