All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Educational theme | Efficacy and safety of camidanlumab tesirine in patients with R/R classical Hodgkin lymphoma
Patients with relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL) have limited therapeutic options available. After relying on chemotherapy for years to treat patients with lymphoma, targeted approaches such as immune checkpoint inhibition and chimeric antigen receptor T-cell therapy have provided high efficacy with tolerable adverse event (AE) profiles. In recent years, chemotherapy-free treatment options have been and continue to be explored, and the Lymphoma Hub has previously reported on advances in these options for the treatment of lymphoid malignancies.
A promising new chemotherapy-free treatment option currently being explored is camidanlumab tesirine, an antibody-drug conjugate comprising a human immunoglobulin G1 anti-CD25 monoclonal antibody conjugated to a pyrrolobenzodiazepine dimer warhead. Camidanlumab tesirine has previously demonstrated encouraging antitumor activity and a manageable safety profile in a phase I trial in patents with R/R cHL. The U.S. Food and Drug Administration (FDA) recently lifted the partial clinical hold on the phase II clinical trial (NCT04052997) of camidanlumab tesirine as reported by the Lymphoma Hub. During the 16th International Conference of Malignant Lymphoma (16-ICML), Professor Pier Zinzani presented the preliminary findings from the same phase II trial of camidanlumab tesirine in patients with R/R cHL. This educational theme article provides a summary of the presentation.
Study design
This is an ongoing phase II multicenter, open-label, single-arm clinical trial evaluating the safety and efficacy of camidanlumab tesirine in patients with R/R cHL. Eligible patients were aged ≥18 years with R/R cHL and had received ≥3 prior lines of systemic therapy, including brentuximab vedotin (BV) and programmed cell death protein 1 (PD-1) blockade, and had measurable disease with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2.
Patients received a 30-minute intravenous infusion of camidanlumab tesirine on Day 1 of each 3-week cycle (Figure 1).
Figure 1. Treatment schema*
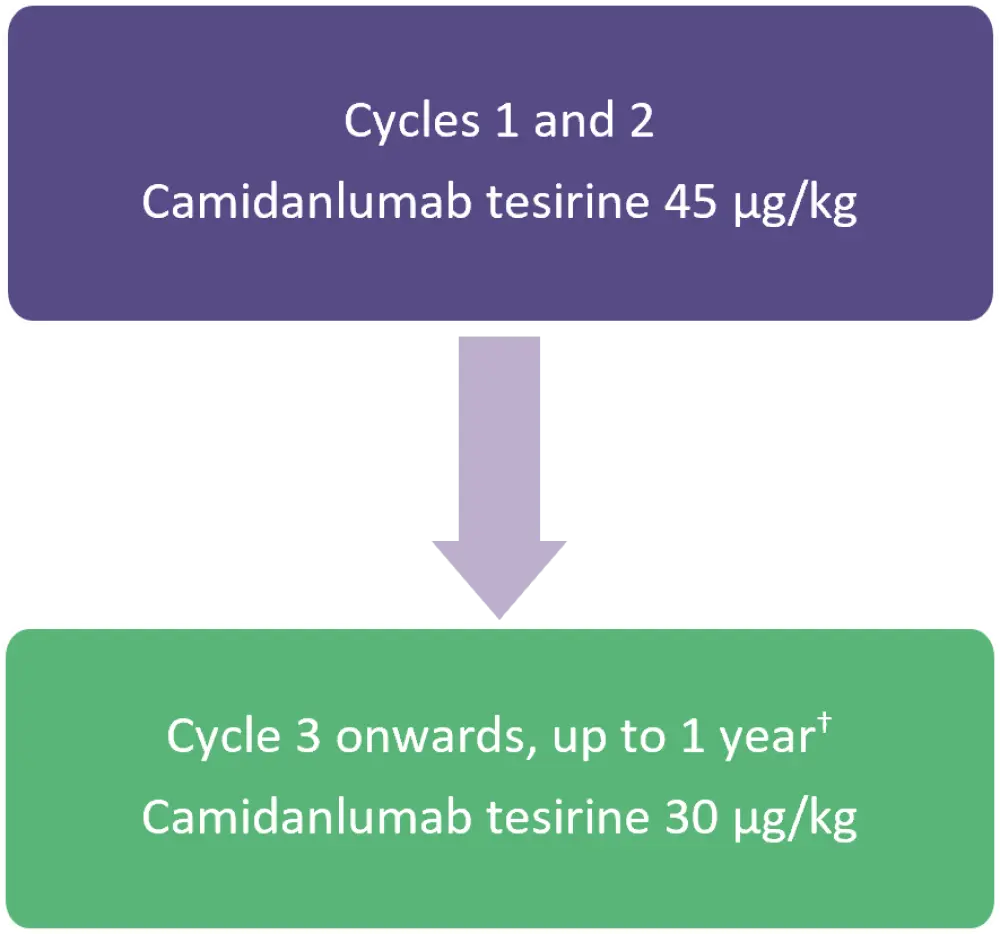
*Adapted from Zinzani, et al.1
†Or until discontinuation due to disease progression, unacceptable toxicity, or other reasons.
- The primary endpoint was efficacy by overall response rate (ORR) per 2014 Lugano classification.
- The secondary endpoints included:
- duration of response (DOR; time from first documentation of tumour response to disease progression or death);
- progression-free survival (time from first dose of study until disease progression or death);
- proportion of patients who receive hematopoietic stem cell transplantation; and
- safety (frequency and severity of AEs).
Baseline characteristics
At data cut off (March 2021), a total of 117 heavily pre-treated patients with a median of six prior lines of systemic therapy (range, 3–19) were included. The median age was 37 years (range, 19–87 years) and 62% of the study population was male. In total, 99% of the patients had received prior BV and PD-1 blockade therapy (Table 1).
Table 1. Baseline characteristics*
|
BV, brentuximab vedotin; cHL, classical Hodgkin lymphoma; ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplantation; PD-1, programmed cell death protein 1; SD, standard deviation. |
|
|
Characteristic, % (unless otherwise stated) |
Total |
|---|---|
|
Histology |
|
|
Nodular sclerosis cHL |
78 |
|
Other/unknown/not evaluable† |
22 |
|
ECOG status |
|
|
0 |
54 |
|
1 |
41 |
|
2 |
5 |
|
Prior therapies |
|
|
BV |
99 |
|
PD-1 blockade therapy |
100 |
|
BV and PD-1 blockade therapy |
99‡ |
|
Autologous HSCT |
50 |
|
Allogenic HSCT |
3 |
|
Autologous and allogenic HSCT |
10 |
|
Number of camidanlumab tesirine cycles, mean (SD) |
5 (3) |
|
Disease status after first-line systemic therapy |
|
|
Relapsed |
66 |
|
Refractory |
25 |
|
Other§ |
9 |
|
Disease status after last-line systemic therapy |
|
|
Relapsed |
33 |
|
Refractory |
56 |
|
Other§ |
11 |
Results
Centrally reviewed ORR
In total, 101 of the 117 patients were available for efficacy evaluation.
- Camidanlumab tesirine demonstrated an ORR of 66% (95% confidence interval, 56–75) with complete response and partial response rates of 28% and 39%, respectively.
- Nine patients discontinued the study due to hematopoietic stem cell transplantation, and one of the nine patients died due to disease progression.
- Median DOR was not reached at a median follow-up duration of 5 months (range, 1–18 months) and median progression-free survival was 9.2 months (range, 9 months to not reached).
- Comparison of ORR at data cut off to the previous analysis from the phase I study2 and the current phase II study prior to data cut-off3 demonstrated a shorter median study duration of 5 months (range, 1–18 months) versus 10 months (range, 0–26 months) and 6 months (range, 2–11 months), respectively (Figure 2).
Figure 1. ORR in context of prior analyses*
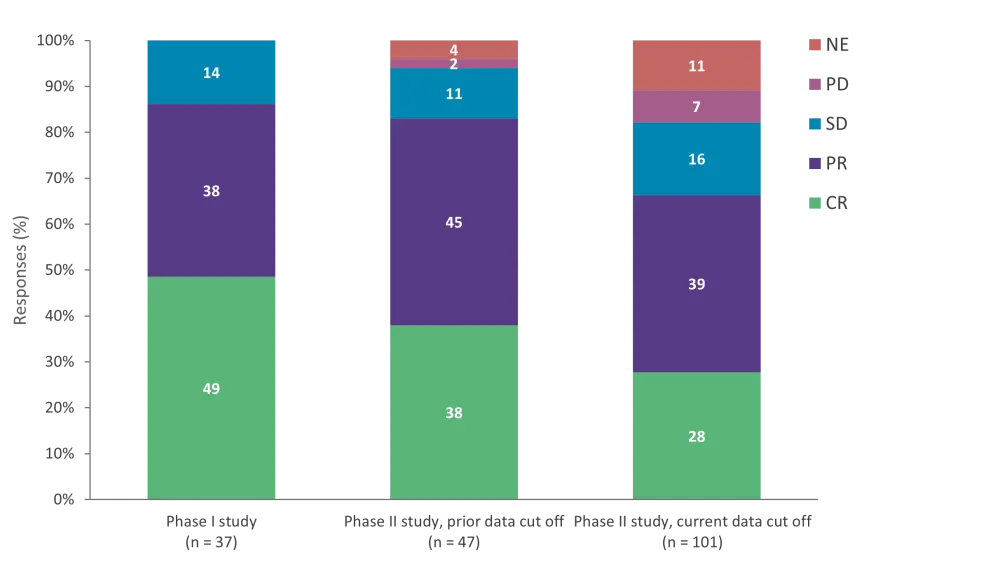
CR, complete response; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
*Adapted from Zinzani, et al.1
TEAEs
Any grade treatment-emergent AEs (TEAEs) occurred in 99% of patients and TEAEs of Grade ≥3 occurred in 53% of patients (Table 2).
Table 2. TEAEs*
|
TEAEs, treatment-emergent adverse events. |
|
|
TEAEs, % |
Total |
|---|---|
|
All grade TEAEs |
|
|
Fatigue |
37 |
|
Maculopapular rash |
28 |
|
Nausea |
27 |
|
Pyrexia |
27 |
|
Asemia |
21 |
|
Grade ≥3 TEAEs |
|
|
Hypophosphatemia |
8 |
|
Maculopapular rash |
7 |
|
Thrombocytopenia |
7 |
|
Anemia |
6 |
|
Lymphopenia |
6 |
|
All grade TEAEs leading to dose delay, reduction, or discontinuation |
|
|
Dose delay or reduction |
48 |
|
Discontinuation |
14 |
- Categories of TEAEs that were considered pyrrolobenzodiazepine-associated included skin reactions (65%), liver function test abnormalities (27%), and edema or effusion (12%).
- Fatal TEAEs were observed in three patients and included myocardial infarction, respiratory failure, and adenovirus infection, although these were considered unlikely to be related to treatment.
- Guillain-Barré syndrome (GBS)/polyradiculopathy was reported in 6% of patients, with Grade 2 GBS in one patient, Grade 2 radiculopathy in one patient, Grade 3 GBS in two patients, Grade 3 polyneuropathy in one patient, and grade 4 GBS in two patients.
- Three of the seven patients with GBS/polyradiculopathy were either recovered or recovering.
Conclusion
This phase II trial demonstrated the efficacy of camidanlumab tesirine in heavily pre-treated patients with R/R cHL post BV and PD-1 blockade failure. The trial also demonstrated encouraging DOR, and the safety profile was consistent with a previous safety assessment of camidanlumab tesirine. However, the occurrence of GBS/polyradiculopathy is a concern and swift countermeasures, such as intravenous immunoglobulin, plasma exchange, or high-dose steroids, are warranted.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

