All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Efficacy and safety of pembro-GVD as second-line therapy for patients with R/R cHL
Featured:
Most patients with classical Hodgkin lymphoma (cHL) are cured with frontline therapy, but some patients with advanced stage cHL develop relapsed or refractory (R/R) disease. Second-line therapy followed by consolidation with high-dose therapy and autologous hematopoietic cell transplant (HDT/AHCT) is the standard approach for these patients. Complete response (CR) attained on fluorodeoxyglucose-positron emission tomography (FDG-PET) or FDG-computer tomography (FDG-CT) before HDT/AHCT is considered a strong positive prognostic factor for long-term outcomes in patients with R/R cHL. Second-line therapies—including platinum- and gemcitabine-based regimens—are associated with high rates of CR (50‒60%) and have well-tolerated safety profiles, though there is no single standard second-line regimen. The addition of brentuximab vedotin (BV), checkpoint inhibitors, and bendamustine to second-line therapy have resulted in improved CR rates (up to 75%), though the increasing use of BV as frontline therapy for cHL makes it a less desirable option in the second line.
Moskowitz, et al.1, building on the previously reported efficacy of programmed cell death protein-1 blockade in patients with cHL, developed a novel second-line regimen using pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin (pembro-GVD) for patients with cHL; they recently published the findings from their phase II trial (NCT03618550) in the Journal of Clinical Oncology. The investigators’ rationale for selecting gemcitabine, vinorelbine, and liposomal doxorubicin (GVD) along with pembrolizumab was that GVD had previously reported high overall response rates (ORR) and each drug from GVD had the potential to stimulate tumor-specific immune responses.1
Study design
This was a single-arm, phase II, investigator-initiated, multicenter trial in transplant eligible patients with R/R cHL following first-line therapy. Eligible patients were aged ≥18 years with Eastern Co-operative Oncology Group (ECOG) performance score of ≤1 and adequate organ function. The treatment schema is presented in Figure 1 and comprised 21-day cycles. Patients were pre-medicated with diphenhydramine, famotidine, and dexamethasone, and liposomal doxorubicin was administered over 2 hours on Day 1 of Cycle 1 to reduce any liposomal doxorubicin infusion-related toxicities.
Response was assessed by FDG-PET/CT after two and four cycles of treatment.
- Patients achieving CR (defined as Deauville ≤3) after two cycles were eligible to proceed to HDT/AHCT (Figure 2). HDT was initiated 3–6 weeks after last cycle of pembro-GVD.
- Patients with less than CR were given two additional cycles of pembro-GVD followed by FDG-PET/CT (Figure 2).
- The primary endpoint was ORR (partial response [PR] or CR). Secondary endpoints included progression-free survival, overall survival, and adverse events (AEs).
Figure 1. Treatment schema*
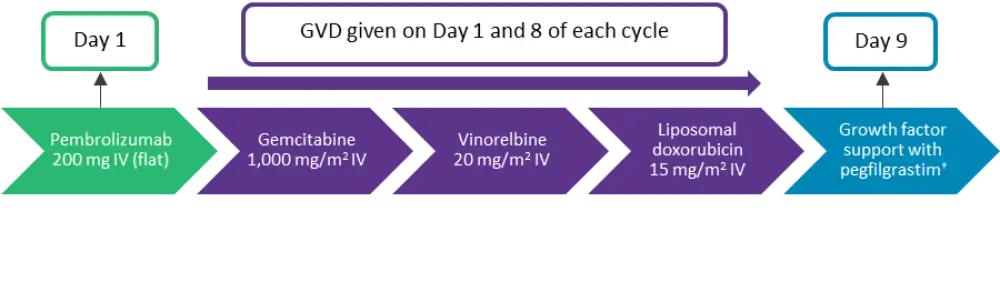
IV, intravenous.
*Derived from Moskowitz, et al.1
†Or equivalent.
Figure 2. Consort diagram*
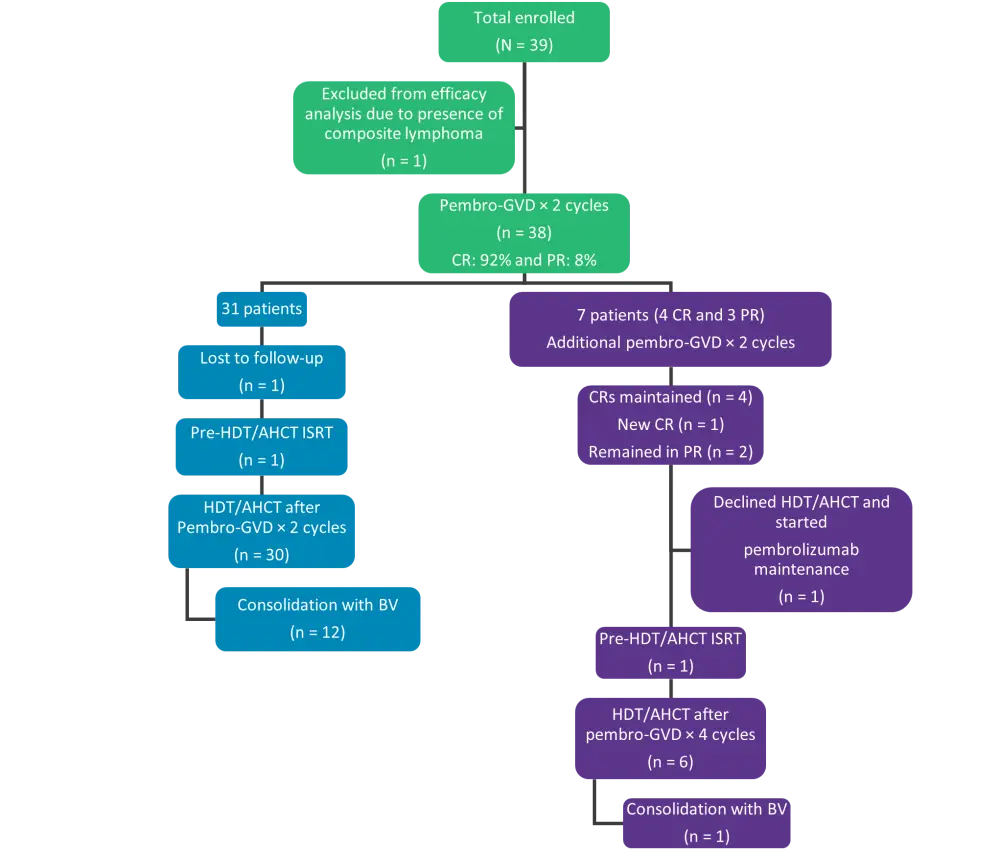
BV, brentuximab vedotin; CR, complete response; HDT/AHCT, high-dose therapy and autologous hematopoietic cell transplantation; ISRT, involved site radiation therapy; pembro-GVD, pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin; PR, partial response.
*Adapted from Moskowitz, et al.1
Results
Baseline characteristics
A total of 39 patients were enrolled; the median age was 38 years (range, 21–71 years) and 46% of the patients were male. Most patients (36%) received doxorubicin, bleomycin, vinblastine, and dacarbazine as frontline therapy. Primary refractory disease was present in 41% and an additional 38% had relapsed within the first year of completing frontline therapy (Table 1).
Table 1. Baseline characteristics*
|
CR, complete response; EBV, Epstein-Barr virus. |
|
|
Characteristics, % |
Total (N = 39) |
|---|---|
|
Disease status (Lugano staging) |
|
|
Initial diagnosis |
|
|
I or II |
26 |
|
III or IV |
74 |
|
Time of enrollment |
|
|
I or II |
41 |
|
III or IV |
59 |
|
Disease characteristics at time of enrollment |
|
|
B-symptoms |
15 |
|
Extranodal disease |
31 |
|
EBV-positive |
13 |
|
Disease status after frontline therapy |
|
|
Refractory (no CR to frontline and progression ≤1 year) |
41 |
|
Relapse (CR to frontline and remission duration ≤1 year) |
38 |
|
Relapse (CR to frontline and remission duration >1 year) |
21 |
Efficacy
All patients were assessed for ORR except one who was not eligible for the efficacy analysis due to the presence of transformed follicular lymphoma plus cHL. Among the 38 evaluable patients, ORR and CR after pembro-GVD were 100% and 95%, respectively (Table 2).
Table 2. Efficacy*
|
CI, confidence interval; CR, complete response; ORR, overall response rate; PR, partial response; pembro-GVD, pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin. |
|||
|
Characteristic, % (95% |
Pembro-GVD × 2 |
Pembro-GVD × 4 |
Pembro-GVD Overall |
|---|---|---|---|
|
ORR |
100 (91–100) |
100 (59–100) |
100 (91–100) |
|
CR |
92 (79–98) |
71 (29–96) |
95 (82–99) |
|
PR |
8 (2–21) |
29 (4–71) |
5 (1–18) |
Eight patients (of which seven were evaluable) received two additional cycles of pembro-GVD (Figure 2). Overall, 36 of the 38 (95%) patients proceeded to HDT/AHCT following two (n = 30) or four (n = 6) cycles of pembro-GVD (Figure 2).
Thirteen of the 36 patients following HDT/AHCT received maintenance therapy with BV (n = 12) or BV plus nivolumab (n = 1, on a clinical trial) (Figure 2). The median follow-up in patients after HDT/AHCT was 13.5 months (range, 2.6–27 months) and all patients continued to be alive and in remission.
Safety
Most AEs were Grade I or 2; the few Grade 3 AEs included transaminitis (n = 4), neutropenia (n = 4), mucositis (n = 2), thyroiditis (n = 1), and rash (n = 1) (Table 3). The most common immune-related AEs were hyperthyroidism, transaminitis and rash.
- Hyperthyroidism was developed within the first two cycles of treatment in five patients, two of whom subsequently recovered, while the other three remained stable on levothyroxine.
- In total, 13% of patients received systemic steroids (prednisone) for transaminitis or Grade 3 rash.
- The difference in the frequency of immune-related events in patients receiving two vs four cycles of pembro-GVD was not significant (p = 0.2).
- Treatment was delayed in 23% of patients for a median of 6 days (range, 4–14 days) and reasons for the delay included transaminitis, rash, mucositis, neutropenia, and upper respiratory infection.
- One patient required a 20% dose reduction of gemcitabine on Cycle 2, Day 8 for transaminitis, while another patient required a 25% dose reduction of GVD on Cycle 4, Day 8 for Grade 3 mucositis.
Table 3. Adverse events
|
AEs, adverse events; AST/ALT, aspartate transaminase/alanine aminotransferase. |
||||
|
AEs |
Grade 1, n |
Grade 2, n |
Grade 3, n |
Total, % (N = 39) |
|---|---|---|---|---|
|
Rash |
13 |
5 |
1 |
49 |
|
Elevated AST/ALT |
5 |
7 |
4 |
41 |
|
Mucositis oral |
5 |
8 |
2 |
38 |
|
Nausea |
12 |
2 |
— |
36 |
|
Fatigue |
11 |
1 |
— |
31 |
|
Headache |
7 |
2 |
— |
23 |
|
Infusion-related |
— |
8 |
— |
21 |
|
Diarrhea |
5 |
2 |
— |
18 |
|
Neutrophil count |
1 |
— |
4 |
13 |
|
Hyperthyroidism |
2 |
2 |
1 |
13 |
|
Vomiting |
4 |
1 |
— |
13 |
|
Hand-foot |
4 |
1 |
— |
13 |
|
Constipation |
3 |
2 |
— |
13 |
Conclusion
This phase II study evaluating the efficacy and safety of pembro-GVD in patients with R/R cHL demonstrated that pembro-GVD was highly effective as second-line therapy and was well tolerated in patients with R/R cHL. In addition, the efficacy of pembro-GVD was comparable with other second-line regimens used in cHL. Pembro-GVD was well-suited for outpatient administration and showed encouraging outcomes in patients previously treated with BV. Further research is warranted into investigating the durability of responses and long-term outcomes with pembro-GVD in patients with R/R cHL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Sheetal Bhurke
Sheetal Bhurke