All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
ESMO 2016 | Educational Session – FL: novel developments beyond chemotherapy
The ESMO 2016 congress took place in Copenhagen, Denmark, on 7–11 October, and is considered the most important European meeting to present oncology research aiming to find the most effective cancer management and treatment strategies today.
During an Educational Session on 8th October, Professor Michele Ghielmini, Medical Director of the Oncology Institute of Southern Switzerland, Bellinzona, and Head of the Medical Oncology Department, gave a talk titled: “Follicular Lymphoma (FL): novel developments beyond chemotherapy”.
Introduction
Professor Ghielmini began by explaining that FL is curable in some cases, specifically:
- Early stage – 50% cured with radiotherapy (RT)
- Grade 3B – 50% cured with R-CHOP
- Allogeneic transplantation – 50% cured with graft versus host disease (GvHD)
The only life-prolonging treatment: rituximab
Data published by Schulz et al., published in the Journal of the National Cancer Institute in 2007, were discussed. Overall Survival (OS) data was available for 1,480 patients across five different trials for the subgroup analysis specifically for FL. 759 of these patients were treated with rituximab in combination with chemotherapy (R-chemo) and 721 received chemotherapy (CT) alone. 97 (12.8%) patients in the R-chemo group and 142 (19.7%) in the CT-alone group died. No heterogeneity existed among the trials (P=.59). The researchers calculated a pooled hazard ratio for mortality of FL patients of 0.63 (95% CI = 0.51–0.79), indicating that OS in the R-chemo group is significantly higher than in the CT-alone group.
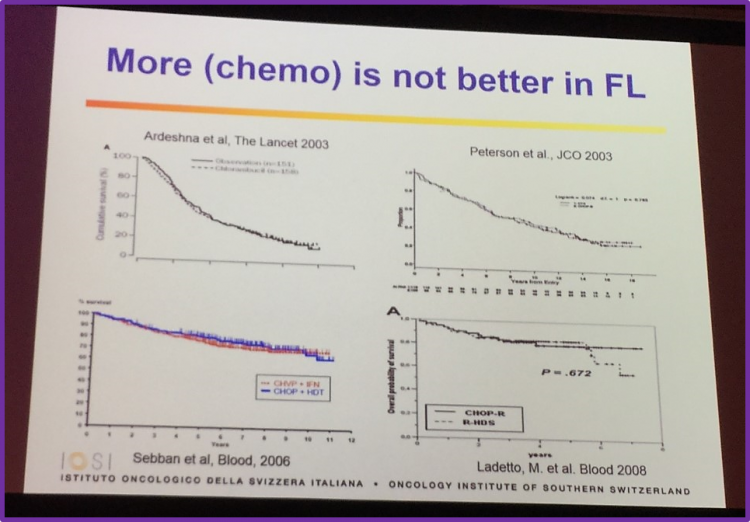
More (CT) is not better in FL
Professor Ghielmini showed data from four different clinical trials (Ardeshna et al. The Lancet 2003; Peterson et al. JCO 2003; Sebban et al.Blood 2006; Ladetto et al. Blood 2008) which found that there is no statistically significant benefit to adding high-dose CT to first-line therapy and high-dose CT should be saved for treating relapses.
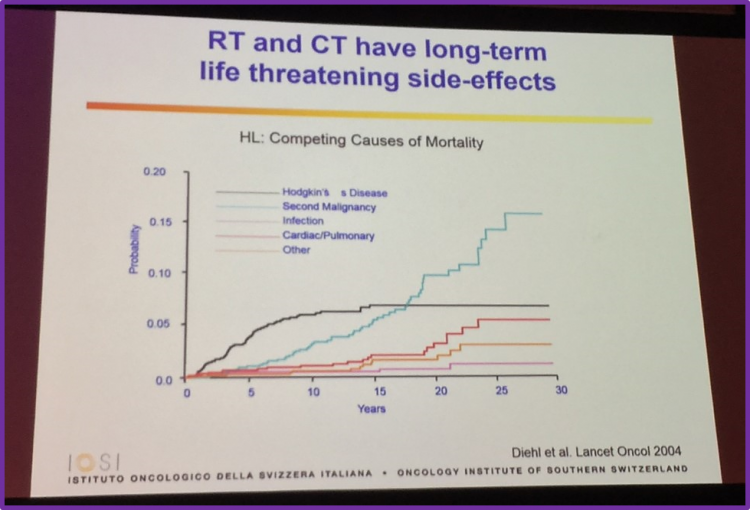
RT and CT have long-term threatening side-effects
Data published by Diehl et al. in Lancet Oncology in 2004 demonstrates that mortality amongst patients with Hodgkin Lymphomas (HL) have many different competing causes including the disease itself, secondary malignancies, infections and cardiac/pulmonary complications.
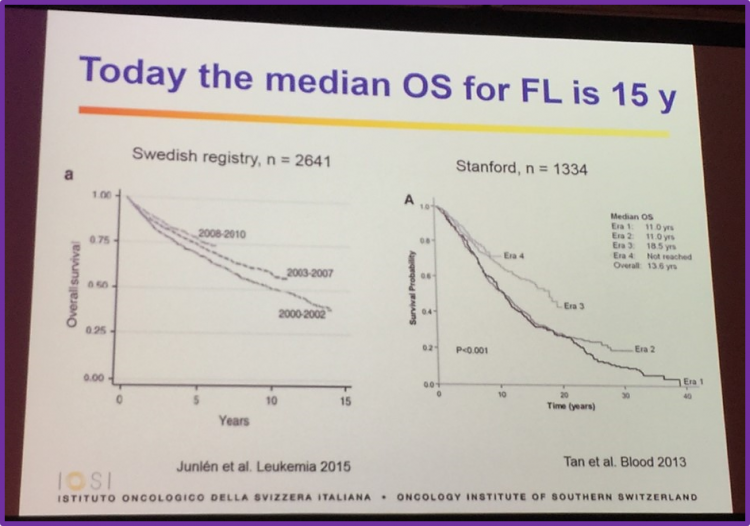
Moreover, 90% of patients with FL aged less than 40 are alive at 10 years, and the median survival of these patients is expected to be more than 30 years. Data published by Conconi et al. in Annals of Oncology in 2015 found that patients with FL younger than 40 have a median OS of 24 years and their outcome improved over time. These patients also has a lower incidence of elevated LDH, high beta2-microglobulin and high risk Follicular Lymphoma International Prognostic Index score. However, bone marrow involvement, bulky disease and disseminated lymphadenopathy was more frequent. At a median follow-up of 10 years, ≤40 year old patients had a significantly higher OS rate (81% vs 51%), cause specific survival rate (82% vs 60%) and Progression Free Survival (PFS; 39% vs 24%) compared to patients aged >40 years. Moreover, Casulo et al. analyzed the National LymphoCare Study to describe characteristics, initial treatments and outcomes in FL patients aged ≤40 years compared to patients aged >40 years. There findings were also published in Annals of Oncology in 2015. They found that patients ≤40 had similar outcomes to patients aged 41–60. With a median follow-up of 8 years, OS at 2, 5 and 8 years was 98% (95% CI 93–99), 94% (95% CI 89–97) and 90% (95% CI 83–94), respectively, for younger patients. Patients ≤40 years old had a PFS of 7.3 years (95% CI 5.6–not reached); PFS at 2 and 5 years was 75% and 62%, respectively.
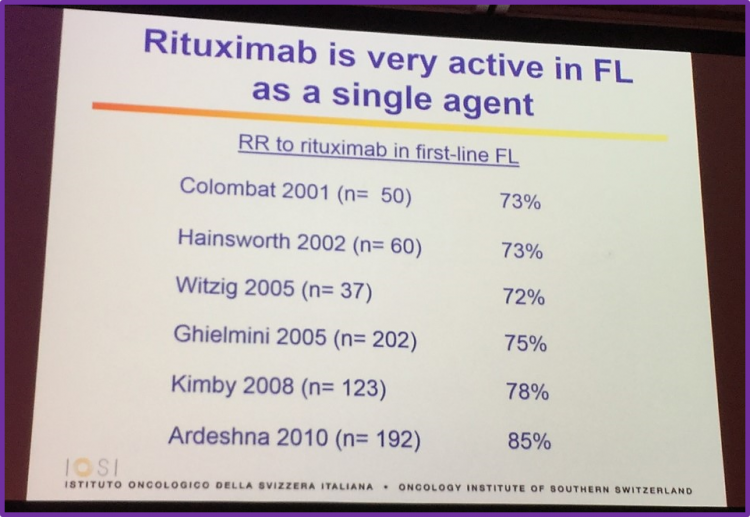
Rituximab as a single agent
The talk then focused on discussing rituximab as first-line treatment for FL, drawing upon much research which resulted in very high Response Rates (RR).
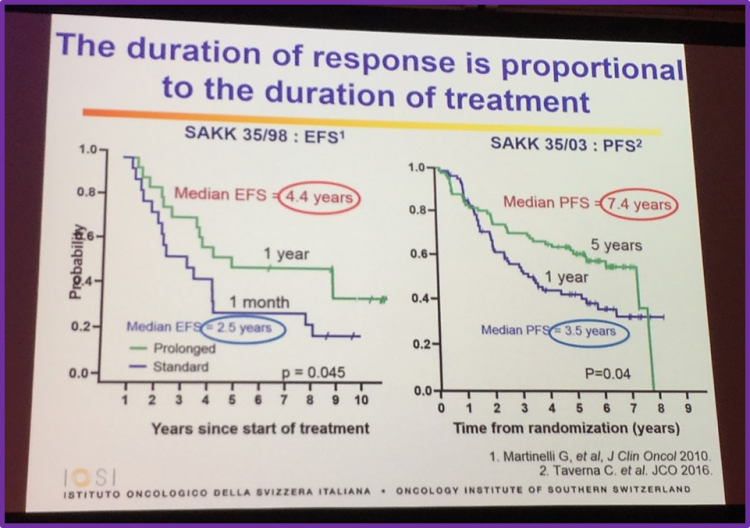
Duration of response is proportional to the duration of treatment
Professor Ghielmini discussed results from two different trials: SAKK 37/98 by Martinelli et al. who published in the Journal of Clinical Oncology in 2010, and the SAKK 35/03 trial by Taverna et al. who also published their findings in the Journal of Clinical Oncology but this year. Martinelli et al. found that at, median follow-up of 9.5 years, the median EFS in the observation arm compared to the prolonged exposure of single-agent rituximab arm was 13 months versus 24 months (P < .001). 5% of patients in the observation arm had no events at 8 years, compared to 27% of prolonged exposure arm. In untreated patients who responded to rituximab induction, 45% had no events at 8 years. This group concluded that a high number of patients experience long-term remission after prolonged exposure to rituximab, especially if no prior treatment had been given and they responded to induction with rituximab. Taverna et al. compared short-term and long-term rituximab maintenance in patients with FL. The SAKK 35/03 trial found that median PFS was 7.4 years (95% CI, 5.1 to not available) in the long-term arm, compared to 3.5 years (95% CI, 2.1 to 5.9) in the short-term arm (log-rank test, P = .04). Taverna et al.conclude that long-term maintenance with rituximab does not improve EFS or OS and was associated with increased toxicity, but does significantly lengthen PFS.
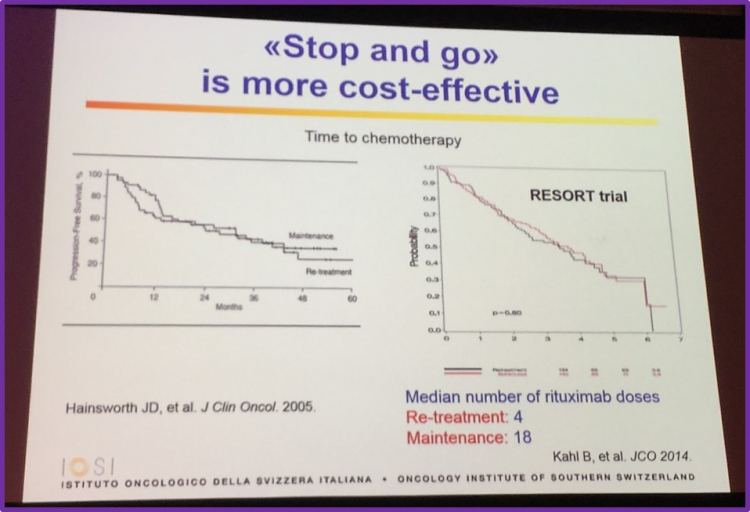
Stop and go is more cost-effective
Hainsworth et al. published the findings of their phase II randomized trial in the Journal of Clinical Oncology in 2005, which aimed to compare the benefit of maintenance rituximab versus rituximab re-treatment at progression in patients with previously treated indolent NHL. It was reported that PFS in the maintenance group was 31.3 months compared to 27.4 months in the re-treatment group. Both treatment approaches were well tolerated, but more maintenance patients remain in continuous remission. Kahl et al. conducted theE4402 (RESORT) trial, a randomized trial designed to compare rituximab maintenance and rituximab re-treatment in patients with low-tumor burden FL. After a median follow-up of 4.5 years, the estimated mean time to treatment failure was 3.9 years and 4.3 years in patients receiving rituximab re-treatment and maintenance, respectively (P = .54). Three-year freedom from cytotoxic therapy in the re-treatment and maintenance groups was 84% and 95%, respectively (P = .03). The median number of rituximab doses was 4 for re-treatment patients and 18 for maintenance patients. The group concluded that re-treatment uses less rituximab doses while providing a similar disease control that maintenance therapy achieves.
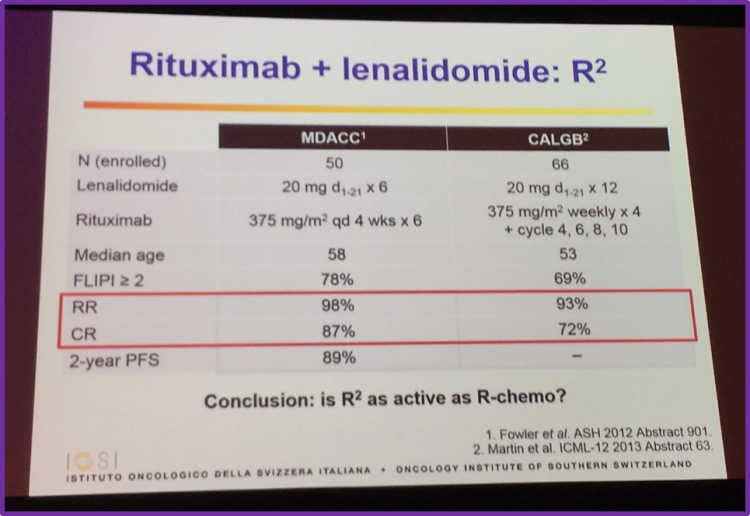
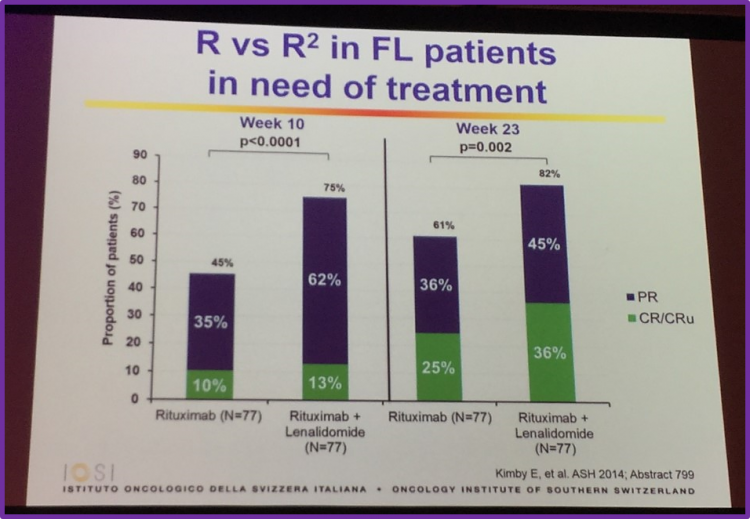
Abstract 799 from ASH 2014, by Kimby et al., was then discussed which included primary endpoint analysis of the randomized phase II trial SAKK 35/10. It was found that the CR/CRu rate was significantly higher in patients receiving R2 than patients just receiving rituximab. This was seen in both the ITT (CR/CRu rate, 36% vs 25%, respectively; P = 0.056) and the per-protocol population (CR/CRu rate, 42% vs 28%, respectively; P = 0.049). However, the group advise that further follow-up is required to observe if the improved response actually translates into increasing time to next treatment and better PFS and OS rates.
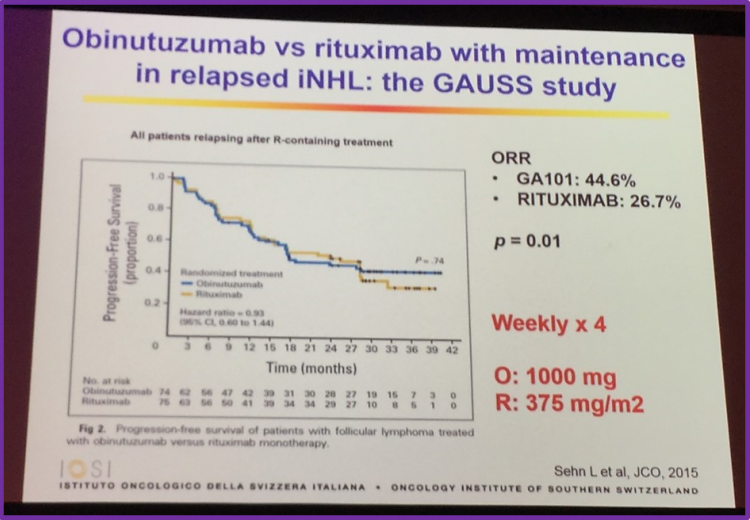
Obinutuzumab versus rituximab with maintenance in relapsed iNHL: the GAUSS study
The talk then sought to answer the question of: are new anti-CD20 therapies any better? Professor Ghielmini began answering this by discussing findings which Sehn et al. published in the Journal of Clinical Oncology in 2015, which concluded that treatment of patients with relapsed CD20+ indolent B-cell NHL with obinutuzumab (GA101) results in a higher ORR.
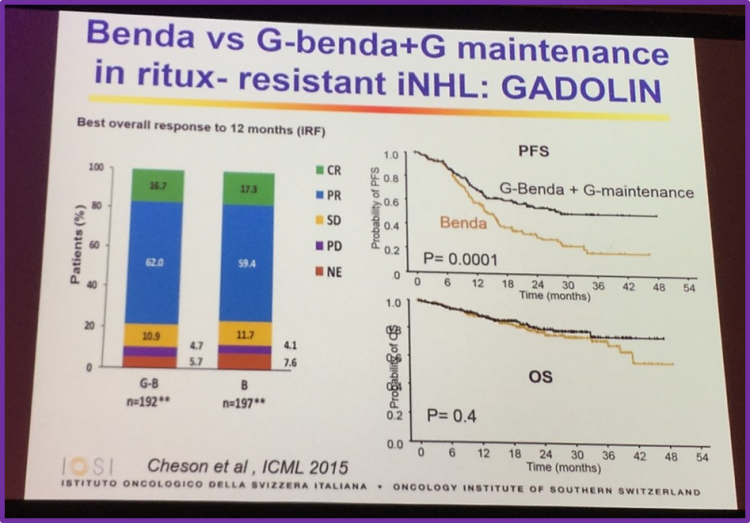
Professor Ghielmini also discussed results of the GADOLIN trial, a phase III study of obinutuzumab plus bendamustine versusbendamustine alone to treat patients with rituximab-refractory indolent NHL. The key conclusions of this trial were:
- Obinutuzumab-bendamustine resulted in a significantly higher clinically meaningful PFS benefit compared to bendamustine monotherapy (not reached vs 14.9 months [HR = 0.55])
- No significant differences in response rates were observed between the two groups
- The incidence of AEs was similar between the two treatment arms
- Obinutuzumab-bendamustine is an effective treatment option for R/R iNHL
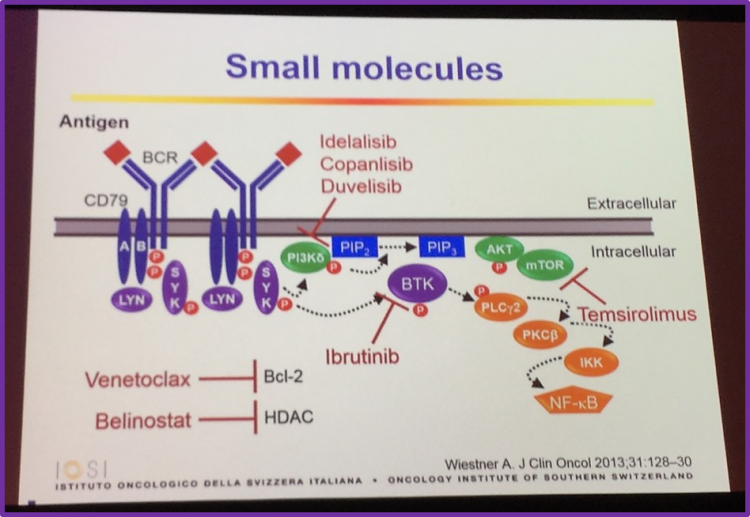
Small Molecules
Professor Ghielmini then discussed small molecules for the treatment of FL, including idelalisib, ibrutinib and venetoclax.
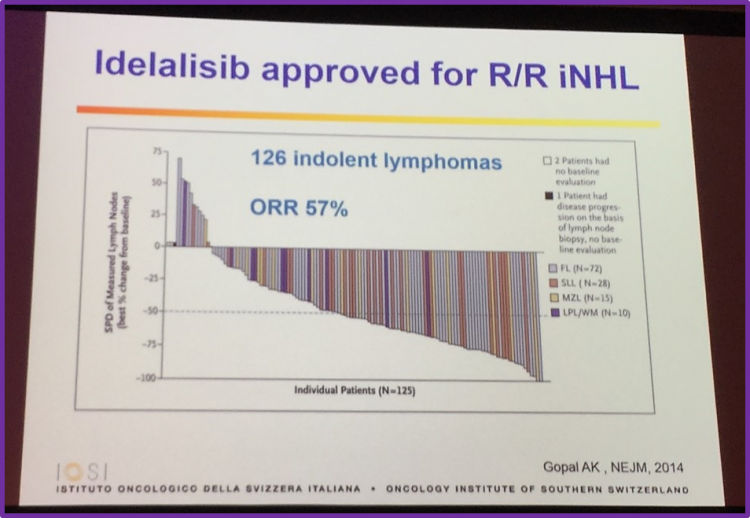
He began with discussion idelalisib, stating it has been approved for the treatment of follicular B-cell NHL. Data published by Gopal et al. in the New England Journal of Medicine in 2014 was also discussed, which found that idelalisib showed anti-tumor activity in patients with iNHL who were refractory to rituximab and alkylating agents, resulting reductions in tumor size in 90% of patients, with 57% meeting the criteria for an objective tumor response.
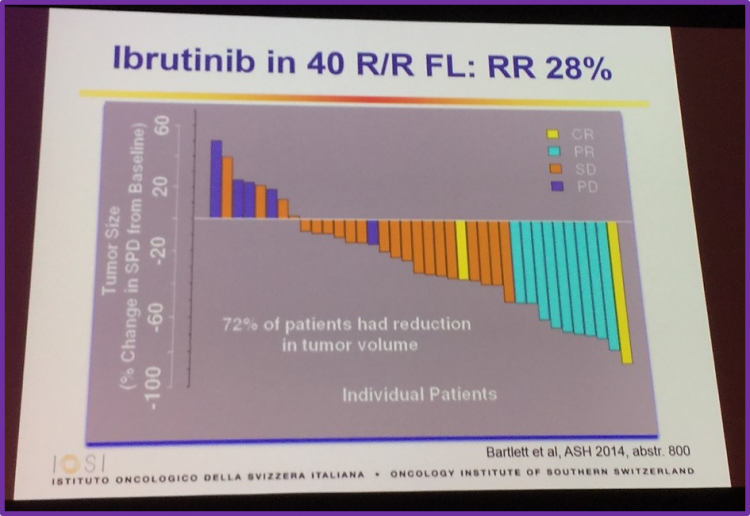
Ibrutinib was the next small molecule covered in this talk, with results by Bartlett et al., who presented at ASH 2014 (abstract 800), being discussed. They found that single agent ibrutinib is tolerated well and demonstrated a modest ORR in patients with R/R FL. At a median follow-up of 6.5 months, the ORR for the 40 ITT patients was 30% (95% CI: 17–47%). Other data discussed included RR and reduction in tumor size (see image below).
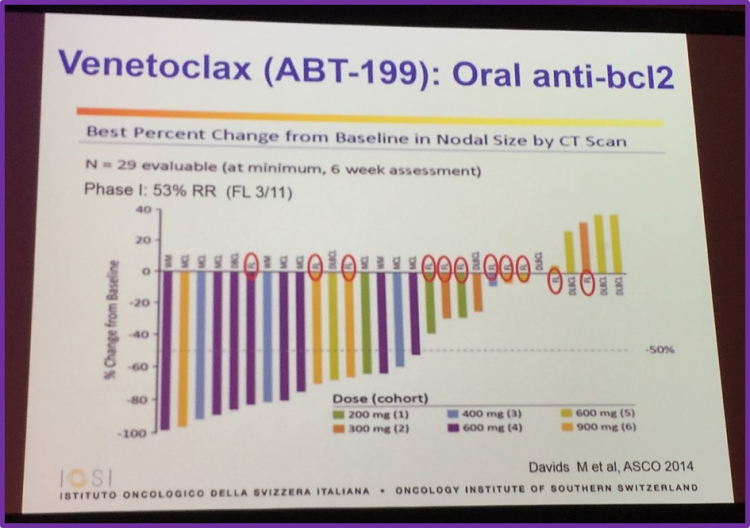
The oral, anti-BCL-2 small molecule venetoclax (ABT-199) was also covered in this talk. Professor Ghielmini covered results of a phase I study by Davids et al., which was presented at ASCO 2014 (abstract 8522), which enrolled 11 FL patients (26%). All responses in FL patients were observed at doses ≥600mg (3/6 FL; 50%). Conclusions of this study were that venetoclax monotherapy demonstrated anti-tumor activity across a range of doses for several NHL subtypes and in FL responses were observed at higher doses.
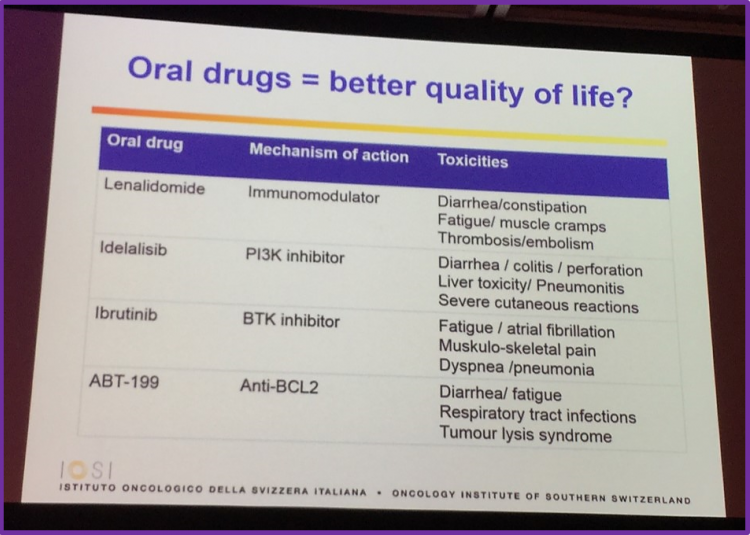
Professor Ghielmini also drew attention to the fact that oral drugs appear to have less severe side effects than other therapies.
Chemo-free combinations
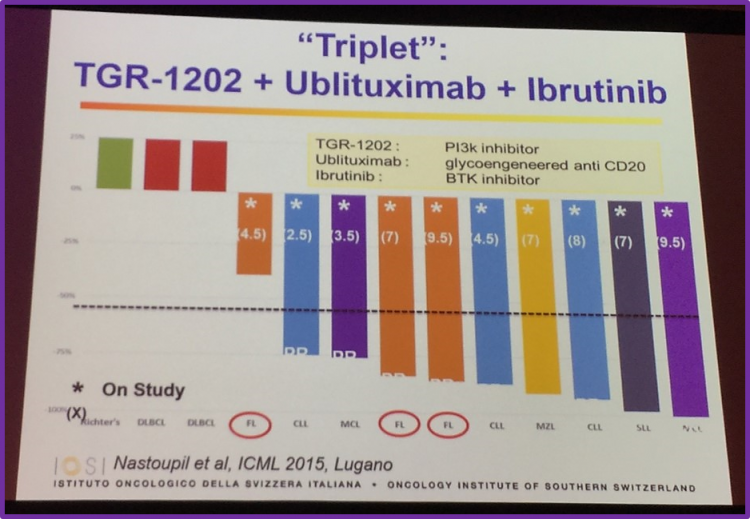
Professor Ghielmini began this topic by discussing the “triplet”: TGR-1202 plus ublituximab plus ibrutinib. Data presented by Nastoupil et al. at ICML 2015, in Lugano, found that the combination of ublituximab, TGR-1202 and ibrutinib had an acceptable safety profile in patients with relapsed B-cell malignancies. In particular, a durable PR of over 9 months was reported in one particular ibrutinib refractory FL patient.
This portion of the talk also covered chemo-free combinations in previously untreated patients with FL:
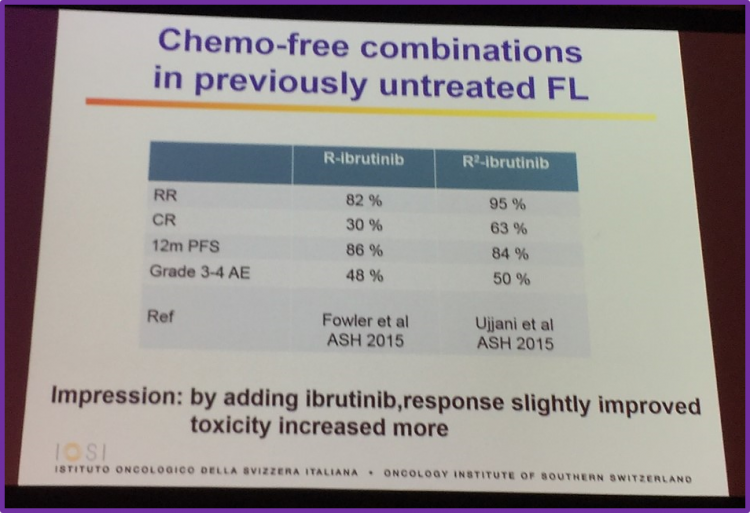
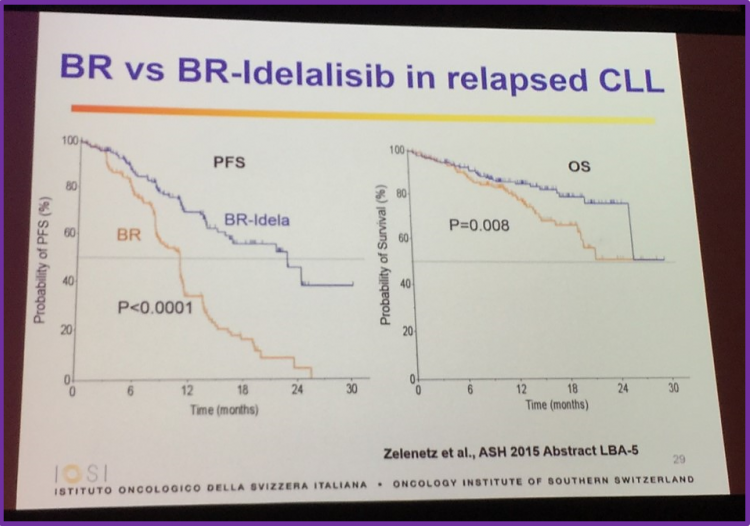
Bendamustine-rituximab versus bendamustine-rituximab-idelalisib
Data produced by Zelenetz et al. was presented at ASH 2015 (abstract LBA-5; NCT01569295) found that idelalisib in combination with bendamustine-rituximab was superior to bendamustine-rituximab alone, increasing PFS and OS. Professor Ghielmini informed the audience of an important drug warning, issued on 11th March 2016, stating that there is an “increased risk of death and serious adverse events, generally due to infections, in patients receiving idelalisib for first line treatment of Chronic Lymphocytic Leukemia (CLL) and relapsed indolent NHL.”
Conclusions
Professor Ghielmini finished his in-depth talk with a succinct conclusion:
- Rituximab single agent is a good alternative to R-chemo in many indolent lymphomas
- There are many non-chemo options, however their therapeutic index is not necessarily better than rituximab or R-chemo
- In future, we need to develop combinations based on the oncogenic alterations of each individual tumor
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

