All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Fixed-duration treatment with ibrutinib and venetoclax in patients with CLL/SLL: Findings from the GLOW and CAPTIVATE trials
Featured:
Bruton’s tyrosine kinase inhibitors are a transformative therapeutic option for patients with high-risk chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), enhancing progression-free survival (PFS) and overall survival (OS). Ibrutinib, a Bruton’s tyrosine kinase inhibitor, and venetoclax are approved in the US and Europe for first-line treatment of CLL. The modes of action of ibrutinib (inhibition of CLL cell proliferation) and venetoclax (targeting of circulating CLL cells) are complementary and synergistic. It is therefore hypothesized that combining ibrutinib and venetoclax will achieve deeper responses in patients with CLL, providing an alternative treatment option.
During the European Hematology Association (EHA)2021 Virtual Congress, Arnon Kater presented results from GLOW (NCT03462719), the first randomized phase III trial of ibrutinib plus venetoclax in patients with CLL/SLL.1 The Lymphoma Hub recently reported an interview with Arnon Kater on the GLOW trial of fixed-duration (FD) ibrutinib and venetoclax as a treatment option for CLL.
CAPTIVATE (NCT02910583) is a phase II trial of ibrutinib plus venetoclax as first-line therapy in patients with CLL/SLL. Results from the minimal residual disease (MRD) cohort of CAPTIVATE were previously reported by the Lymphoma Hub. The primary analysis of the FD cohort from the CAPTIVATE trial was presented during the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting by Paolo Ghia.2 This article summarizes key findings from the GLOW and CAPTIVATE trials.
GLOW (NCT03462719)1
Study design
The phase III GLOW trial enrolled 211 patients with previously untreated CLL/SLL. Patients were ≥65 years of age or 18–64 years of age and unfit (cumulative illness rating scale >6 or creatinine clearance <70 mL/min). Eligible patients had Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2 and no del(17p) or known TP53 mutations and were stratified by immunoglobulin heavy chain variable region gene (IGHV) status and presence of del(11q). Patients were randomized as shown in Figure 1.
The primary endpoint was PFS assessed by independent review committee and secondary endpoints were undetectable MRD (uMRD) in bone marrow (BM) and peripheral blood (PB), complete response (CR) or complete response with incomplete bone marrow recovery (CRi) rate, overall response rate (ORR), OS and safety.
Figure 1. Treatment schema*
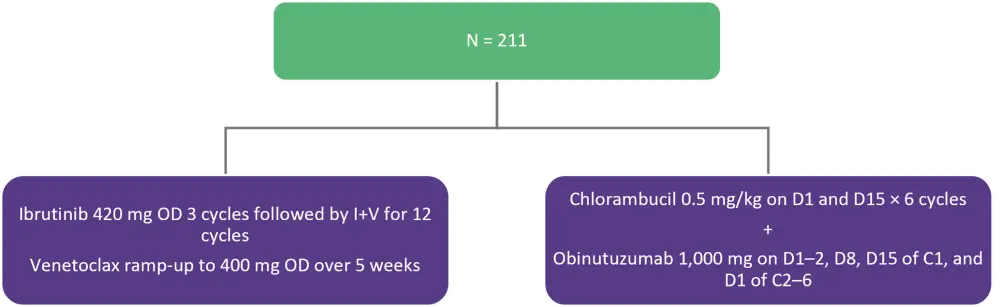
C, cycle; D, day; ; I+V, ibrutinib + venetoclax; OD, once daily.
*Adapted from Kater
Baseline characteristics
The median age was 71 years (range, 47–93 years) and >50% patients in each arm were male. Patient characteristics are shown in Table 1.
Table 1. Baseline characteristics*
|
CIRS, cumulative illness rating scale; Clb+O, chlorambucil + obinutizumab; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; I+V, ibrutinib + venetoclax; IGHV, immunoglobulin heavy chain variable region gene; IQR, interquartile range; LDH, lactate dehydrogenase. |
||
|
Characteristic, % (unless otherwise stated) |
I+V (n = 106) |
Clb+O (n = 105) |
|---|---|---|
|
Median age (range), years |
71 (47–93) |
71 (57–88) |
|
ECOG PS 1–2 |
67 |
63 |
|
Median CIRS score (IQR) |
9 (6–12) |
8 (5–10) |
|
>6 |
70† |
58† |
|
Median CrCL (range), mL/min |
67 (34–168) |
63 (32–181) |
|
Rai stage III–IV |
57 |
53 |
|
Bulky disease ≥5 cm |
39 |
36 |
|
Elevated LDH |
33† |
49† |
|
Mutated IGHV |
26 |
26 |
|
Unmutated IGHV |
52 |
51 |
|
Del(11q) |
19 |
17 |
|
TP53 mutation |
7 |
2 |
Results
Efficacy
- PFS was superior in the ibrutinib + venetoclax (I+V) arm compared with the chlorambucil + obinutuzumab (Clb+O) arm (p < 0.0001) at a median follow-up of 28 months.
- I+V reduced the risk of progression or death by 78% compared with Clb+O.
- Median PFS was not reached in the I+V arm and was 21 months (95% confidence interval, 17–25) in the Clb+O arm.
- Improved rates of PFS in the I+V arm were consistent across all subgroups.
- uMRD rates were higher in the I+V arm compared with the Clb+O arm in BM (52% vs 17%; p < 0.0001) and PB (55% vs 39%; p = 0.0259). At the end of treatment, 85% of patients with uMRD retained their status at 1 year.
- I+V also demonstrated a significantly higher CR/CRi rate compared with Clb+O (39% vs 11%; p < 0.0001) with 90% of responders maintaining their response after 2 years.
Safety
- Safety profiles in both arms were aligned with existing known safety profiles of ibrutinib, venetoclax, and Clb+O. The most common Grade ≥3 adverse events (AEs) were neutropenia (35%), diarrhea (10%), and hypertension (8%) for the I+V arm, and neutropenia (50%), thrombocytopenia (20%), pneumonia (6%), and tumor lysis syndrome (TLS; 6%) for the Clb+O arm.
- Median exposure to treatment was 14 months in the I+V arm and 5 months in the Clb+O arm.
- Ibrutinib lead-in cycle demonstrated effective tumor debulking with only 2% of patients at risk for TLS at the start of venetoclax.
CAPTIVATE (NCT02910583)2
Study design
CAPTIVATE is a multicenter phase II trial evaluating I+V as first-line therapy in patients with CLL/SLL. The study design and patient disposition for the FD cohort, which included patients ≤70 years of age, are presented in Figure 2. Patients with disease progression at follow-up could be retreated with single-agent ibrutinib or fixed-duration I+V.
- The primary endpoint was investigator assessed CR/CRi rate in patients without del(17p).
- The secondary endpoints included ORR, duration of response, uMRD rates, PFS, OS, TLS risk reduction, and safety.
Figure 1. Treatment schema and patient disposition*
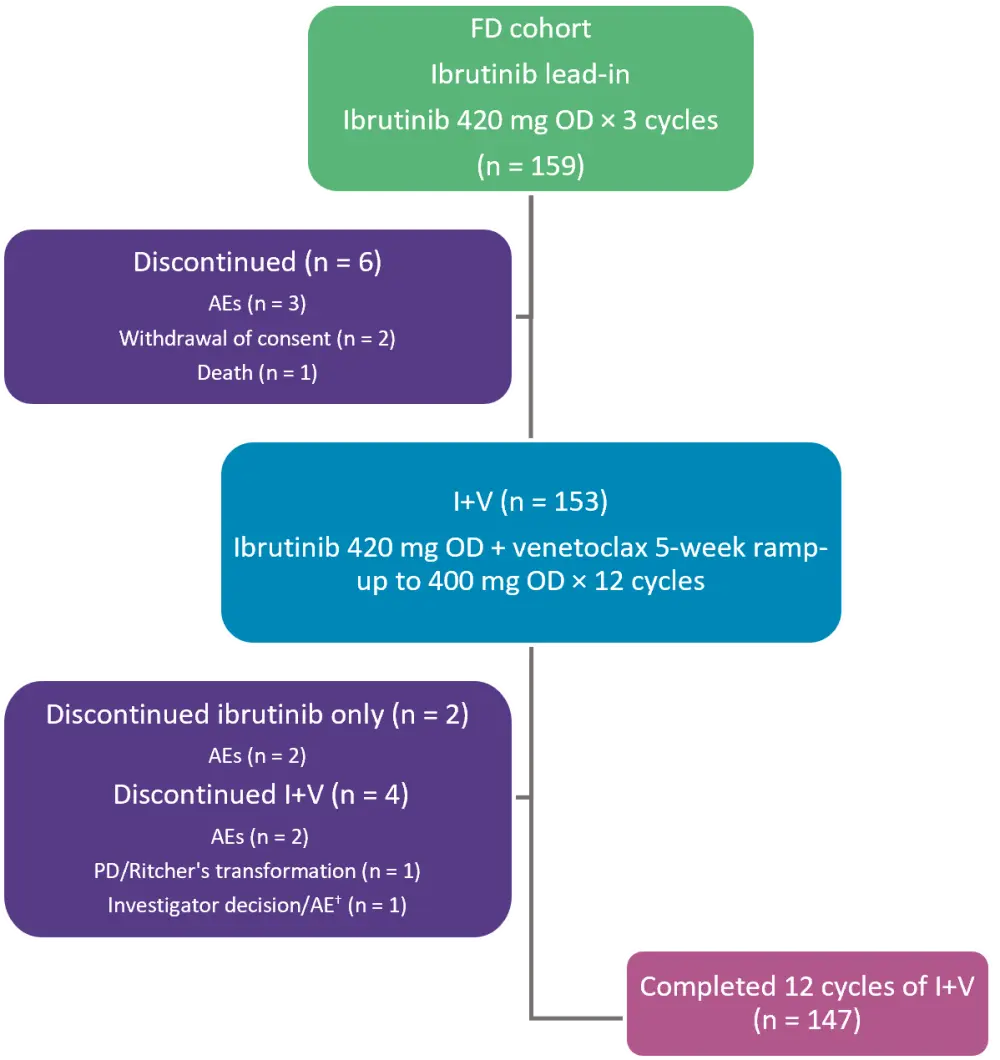
AEs, adverse events; FD, fixed-duration; I+V, ibrutinib + venetoclax; OD, once daily; PD, progressive disease.
*Adapted from Ghia, et al.2
†Patient discontinued ibrutinib due to investigator decision and eventually discontinued venetoclax due to AE.
Baseline characteristics
The total number of patients included in the FD cohort was 159 (all treated), with 136 patients without del(17p). The median age was 60 years (range, 33–71 years) and 67% of patients were male. Patient characteristics are presented in Table 3.
Table 3. Baseline characteristics*
|
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; IGHV, immunoglobulin heavy chain variable region gene. |
|
|
Characteristic, % (unless otherwise stated) |
All treated patients |
|---|---|
|
Rai stage III–IV |
28 |
|
High-risk characteristics |
|
|
Unmutated IGHV |
56 |
|
Del(17p)/TP53 mutation |
17 |
|
Del(17p) |
13 |
|
Del(11q)† |
18 |
|
Complex karyotype‡ |
19 |
|
Any cytopenia |
34 |
|
ANC ≤1.5 × 109/L |
8 |
|
Hemoglobin ≤11 g/dL |
23 |
|
Platelets ≤100 × 109/L |
13 |
|
Lymph node diameter ≥5 cm |
30 |
|
Median ALC (range), × 109/L |
77 (1–503) |
|
ALC ≥25 × 109/L |
75 |
Results
Efficacy
- The primary endpoint of CR/CRi rate was met in 56% of patients without del(17p), significantly surpassing the minimum rate of 37% (p < 0.0001).
-
- ORR (CR/CRi/partial response) was 96% in patients without del(17p) and in all treated patients.
- Duration of CR ≥12 cycles was 87% in patients without del(17p) and 89% in all treated patients.
- High rates of CR were observed across all subgroups except for patients with bulky disease, who had a CR rate of 31% compared with the 66% CR rate in patients without bulky disease.
- uMRD rates were high in both BM (62% vs 60%) and PB (76% vs 77%) in patients without del(17p) and in all treated patients, respectively.
- Similar rates of uMRD were observed in patients with and without bulky disease in BM (63 vs 59%) and PB (both 77%).
- Higher rates of uMRD were observed in patients with unmutated IGHV compared with mutated IGHV in BM (64% vs 53%) and PB (84% vs 67%), respectively.
- At a median follow-up of 28 months, the 24-month PFS rate was 96% and OS was 98% in patients without del(17p).
- Ibrutinib lead-in cycle demonstrated effective debulking of the tumor with 94% (32/34) of patients shifting from high to medium or low tumor burden category.
Safety
- The most frequent any-grade AEs were diarrhea (62%), nausea (43%), neutropenia (42%). and arthralgia (33%); these were also the most frequent Grade 1 or 2 AEs as shown in Table 4.
- I+V was well tolerated with concomitant medications, including antihypertensives, acid reducing agents, antiplatelet agents, and anticoagulants.
Table 4. AEs in all treated patients*
|
AE, adverse event; I+V, ibrutinib + venetoclax. |
|
|
AE, % |
All treated patients |
|---|---|
|
Grade 3 or 4 AEs (≥5%) |
62 |
|
Neutropenia |
33 |
|
Infections† |
8 |
|
Hypertension |
6 |
|
Neutrophil count decreased |
5 |
|
AEs of clinical interest (any grade) |
|
|
Atrial fibrillation |
4 |
|
Major hemorrhage† |
2 |
Re-treatment data from MRD and FD cohorts
Eight patients progressing after FD treatment have been retreated with single agent ibrutinib, six of whom have had a partial response.
Conclusion
The GLOW and CAPTIVATE trials have both demonstrated that FD I+V can elicit durable responses and favorable safety profiles in patients with CLL/SLL. Findings from the CAPTIVATE-FD trial support the efficacy of I+V in most young, fit patients with CLL/SLL, while findings from GLOW demonstrated I+V efficacy in older patients with high-risk features. Taken together, findings from both trials support FD treatment with I+V across a broad range of patients with previously untreated CLL/SLL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Arnon Kater
Arnon Kater