All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Interim results from ZUMA-12: Axi-cel as frontline treatment for high-risk LBCL
Featured:
Patients with high-risk large B-cell lymphoma (LBCL) are mainly treated with rituximab-based chemoimmunotherapies that do not provide sufficient efficacy, as approximately 50% of these patients will not achieve long-term disease remission.1 The CD19 targeting CAR T product, axicabtagene ciloleucel (axi-cel), has shown great success in the relapsed/refractory (R/R) LBCL setting and has already been approved both in Europe and the US for the treatment of adult patients with R/R diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and primary mediastinal B-cell lymphoma (PMBCL) after ≥ 2 lines of prior therapy.1 The phase II trial, ZUMA-12 (NCT03761056), is currently assessing the efficacy and safety of axi-cel as frontline therapy for adults with high-risk LBCL. During the 62nd American Society of Hematology (ASH) Annual Meeting & Exposition, the interim results from ZUMA-121 were presented by Sattva Neelapu and are hereby summarized.
Study design
- A multicenter, open-label, single-arm, phase II trial in adult patients with high-grade confirmed LBCL with MYC and BCL2 and/or BCL6 translocations or an International Prognostic Index (IPI) ≥ 3 at any time prior enrolment. Eligible patients needed to have a positive positron interim emission tomography (PET; Deauville score of 4 or 5) scan after two cycles of anti-CD20 monoclonal antibody plus an anthracycline-containing regimen. The full study design of ZUMA-12 is shown in Figure 1
- Prior to axi-cel infusion, on Days −5, −4, and −3, fludarabine (30 mg/m2), and cyclophosphamide (500 mg/m2) were administered as conditioning chemotherapy
- Non-chemotherapy bridging therapy was optional
- Axi-cel infusion was performed as a single intravenous dose of 2 × 106 CAR T cells/kg on Day 0
- Primary endpoint was complete response (CR) rate as defined by the Lugano classification,2 while secondary outcomes included overall response rate (ORR), duration of response (DoR), event-free survival (EFS), overall survival (OS), progression-free survival (PFS), safety, and the number of circulating CAR T cells and cytokines
Figure 1. ZUMA-12 study design1
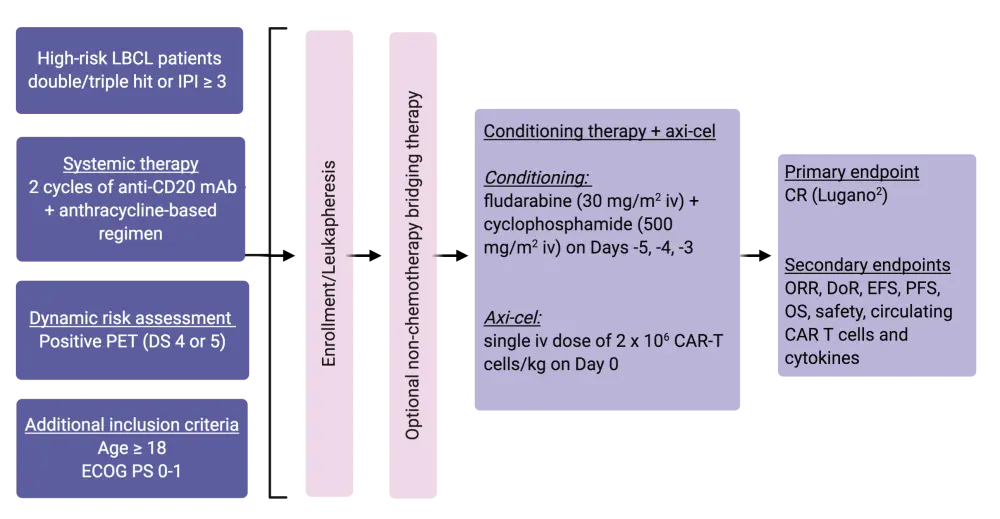
Axi-cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor; CR, complete response; DoR, duration of response; DS, Deauville score; ECOG PS, Eastern Cooperative Oncology Group performance score; EFS, event-free survival; IPI, International Prognostic Index; iv, intravenous; LBCL, large B-cell lymphoma; mAb, monoclonal antibody; ORR, overall response rate; OS, overall survival, PET, positron emission tomography; PFS, progression-free survival.
Results
- In total, 32 out of 37 enrolled patients received an axi-cel infusion. At data cutoff (August 25, 2020), 32 patients were evaluable for safety with a median follow-up of 9.5 months (range, 0.9–18) and 27 patients were included in the efficacy analysis with a median follow-up of 9.3 months (range, 0.9–18)
- Key patient baseline characteristics are shown in Table 1
Table 1. Key patient baseline characteristics in ZUMA-121
|
ECOG PS, Eastern Cooperative Oncology Group performance score; FISH, fluorescence in situ hybridization; IPI, International Prognostic Index. *Determined by FISH per investigator. |
|
|
Baseline characteristic |
N = 32 |
|---|---|
|
Age |
|
|
Median (range), years |
61 (23–86) |
|
≥ 65 years, n (%) |
13 (41) |
|
Male, n (%) |
23 (72) |
|
Disease stage III–IV, n (%) |
28 (88) |
|
ECOG PS ≥ 1, n (%) |
21 (66) |
|
One prior systemic line, n (%) |
32 (100) |
|
Double-/triple-hit*, n (%) |
17 (53) |
|
IPI ≥ 3, n (%) |
23 (72) |
|
Deauville score, n (%) |
|
|
4 |
16 (50) |
|
5 |
16 (50) |
- The following outcomes were reported:
- ORR: 85%
- CR: 74% (n = 20)
- Partial response (PR): 11% (n = 3)
- Stable disease (SD): 15%
- None progressed
- At data cutoff, 19 patients had ongoing responses (70%)
- Median time to CR was 1 month (range, 0.9–6.4), similar to the median time to objective response (1 month [range, 0.9–3.1])
- Five patients (19%) converted from PR (n = 4) or SD (n = 1) to CR
- At a median follow-up of 9.5 months, median DoR, PFS, and OS were not reached
- CAR T-cell expansion was higher in this trial when compared with ZUMA-1, with a median time to peak circulating CAR T cells of 8 days
With regards to safety:
- The most common treatment-related Grade ≥ 3 adverse events were:
-
- Encephalopathy (16%)
- Alanine aminotransferase increase (9%)
- Neutrophil count decrease (9%)
- One death occurred during treatment due to COVID-19
- All 32 patients developed cytokine release syndrome (CRS) while 22 (69%) experienced neurological events (NE). Details regarding grade, time to onset, duration, and management of these events are shown below in Table 2
- The median levels of serum cytokines, including interleukin 6 (IL-6), IL-5, IL-8, interferon γ (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) among others, were associated with Grade ≥ 3 NE or CRS events
Table 2. CRS and NE events in ZUMA-121
|
CRS, cytokine release syndrome; NE, neurological events. *The two unresolved NE events at data cutoff were Grade 1 tremor and Grade 1 memory impairment. |
||
|
Parameter |
CRS (N = 32) |
NE (N = 32) |
|---|---|---|
|
Any Grade, n (%) |
32 (100) |
22 (69) |
|
Grade ≥ 3, n (%) |
3 (9) |
8 (25) |
|
Grade 4, n (%) |
0 (0) |
2 (6) |
|
Grade 5, n (%) |
0 (0) |
0 (0) |
|
Most commonly associated any-grade symptoms, n (%) |
Pyrexia: 32 (100) Chills: 8 (25) Hypotension: 8 (25) |
Encephalopathy: 10 (31) Confusional state: 9 (28) |
|
Management, n (%) |
|
|
|
Tocilizumab |
17 (53) |
0 (0) |
|
Steroids |
8 (25) |
11 (34) |
|
Median time to onset, days (range) |
4 (1–10) |
9 (2–44) |
|
Median duration of events, days (range) |
6 (1–13) |
6 (1–54) |
|
Patients with resolved events, n/N (%) |
32/32 (100) |
20/22 (91)* |
Conclusions
The phase II ZUMA-12 trial is the first to assess the use of CAR T cells as frontline treatment for high-grade LBCL. The interim data show promising response outcomes, with axi-cel infusion leading to an ORR of 85% and a CR rate of 74%. At data cutoff, 70% of patients had ongoing responses, indicating that these responses were durable. Lastly, axi-cel was well tolerated in this patient subset, with manageable CRS and NE toxicities. The authors also highlighted the higher CAR T expansion rates observed in ZUMA-12 compared with ZUMA-1, where axi-cel was used in the R/R setting, potentially indicating an improved CAR T cell fitness when axi-cel is used as first-line treatment. Further data are anticipated to validate these results.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Sattva Neelapu
Sattva Neelapu