All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
iwCLL 2017 | Clinical approach to the diagnosis and initial evaluation of a CLL patient
Session four at iwCLL 2017 took place on 14th May 2017, and was titled “Strategies for the Evaluation and Treatment of CLL in the Front-line Setting.” The session was jointly chaired by Stephen Mulligan (University of Sydney, Royal North Shore Hospital) and Neil E. Kay (Mayo Clinic).
The first of two talks in this session was given by Davide Rossi, MD, from the Institute of Oncology Research and Oncology Institute of Southern Switzerland, Bellinzona, Switzerland. His talk was titled “Clinical Approach to the Diagnosis and Initial Evaluation of a CLL Patient.”
CLL is split into three subgroups: clinically suspected, symptomatic, and asymptomatic. It is a genetically heterogeneous disease and lacks disease defining genetic lesions.
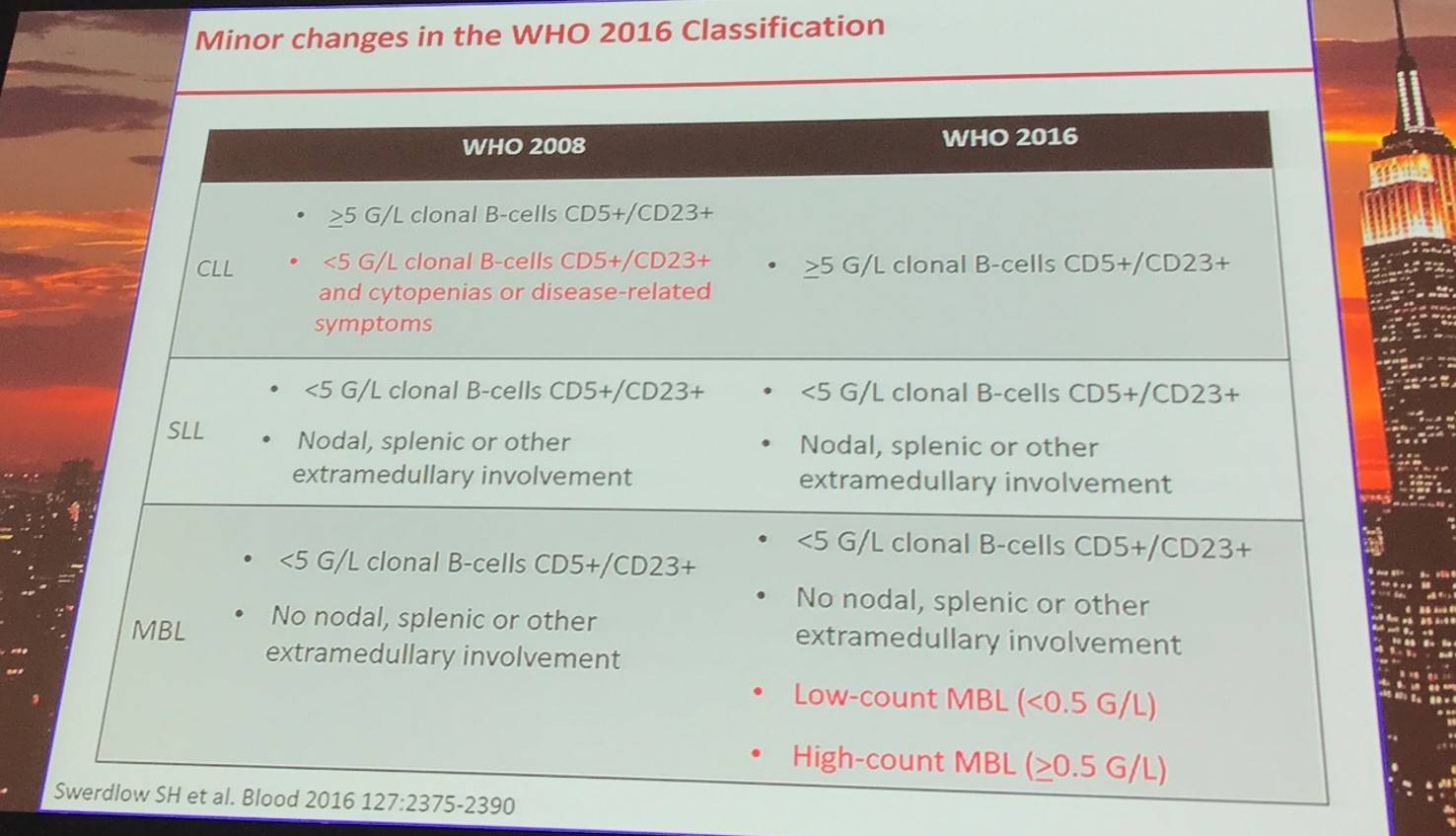
According to the WHO 2016 classification of Richter syndrome, clinical suspicions of transformation include:
- Asymmetric growth of localized lymph nodes
- Bulky disease
- B symptoms
- Sudden and excessive rise in levels of LDH
In terms of PET/CT in Richter syndrome diagnosis:
|
Max SUV cut-off = 5 |
|
|
|
RS |
|---|---|
|
Sensitivity |
91% |
|
Specificity |
80% |
|
Positive predictive value |
53% |
|
Negative predictive value |
97% |
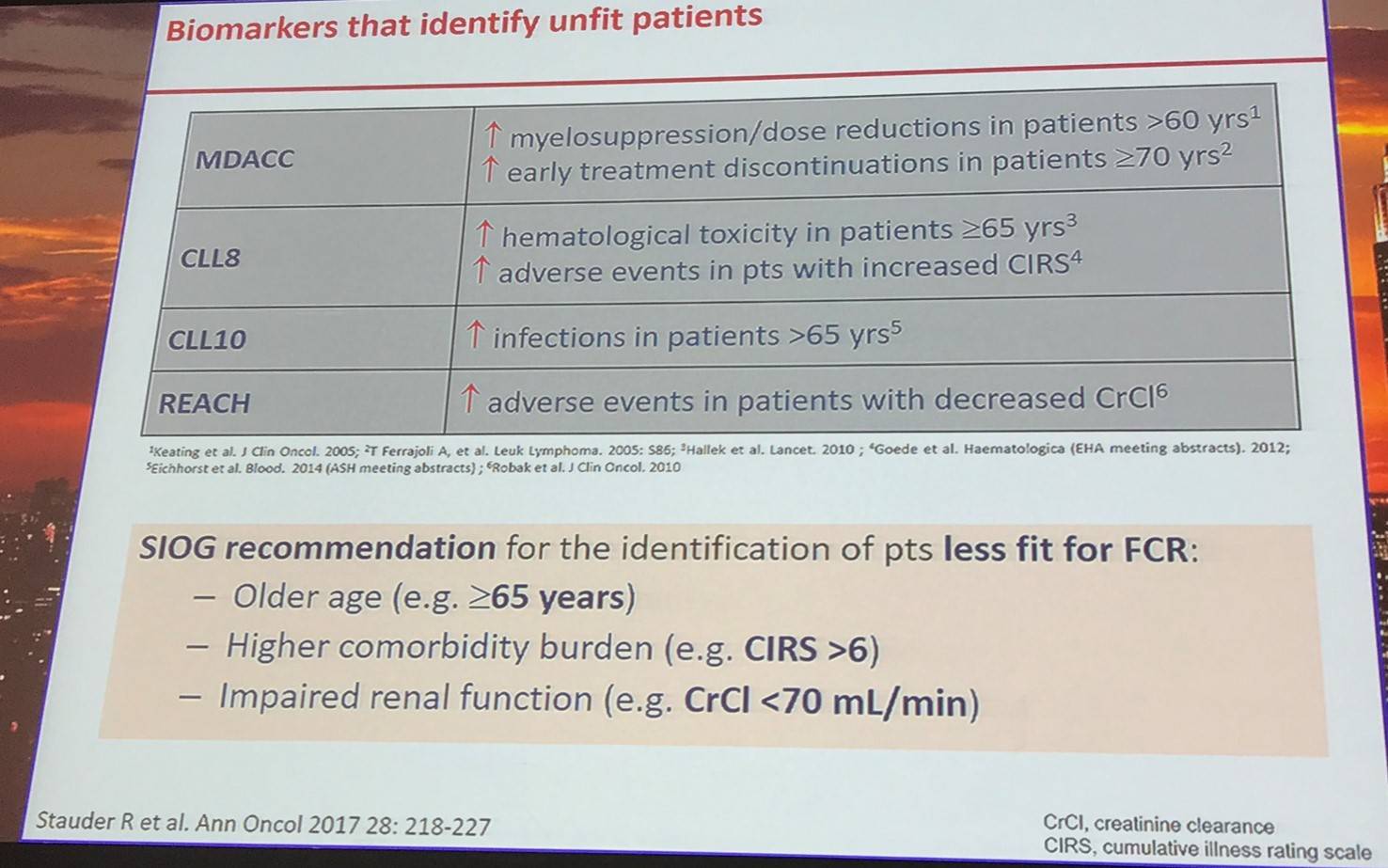
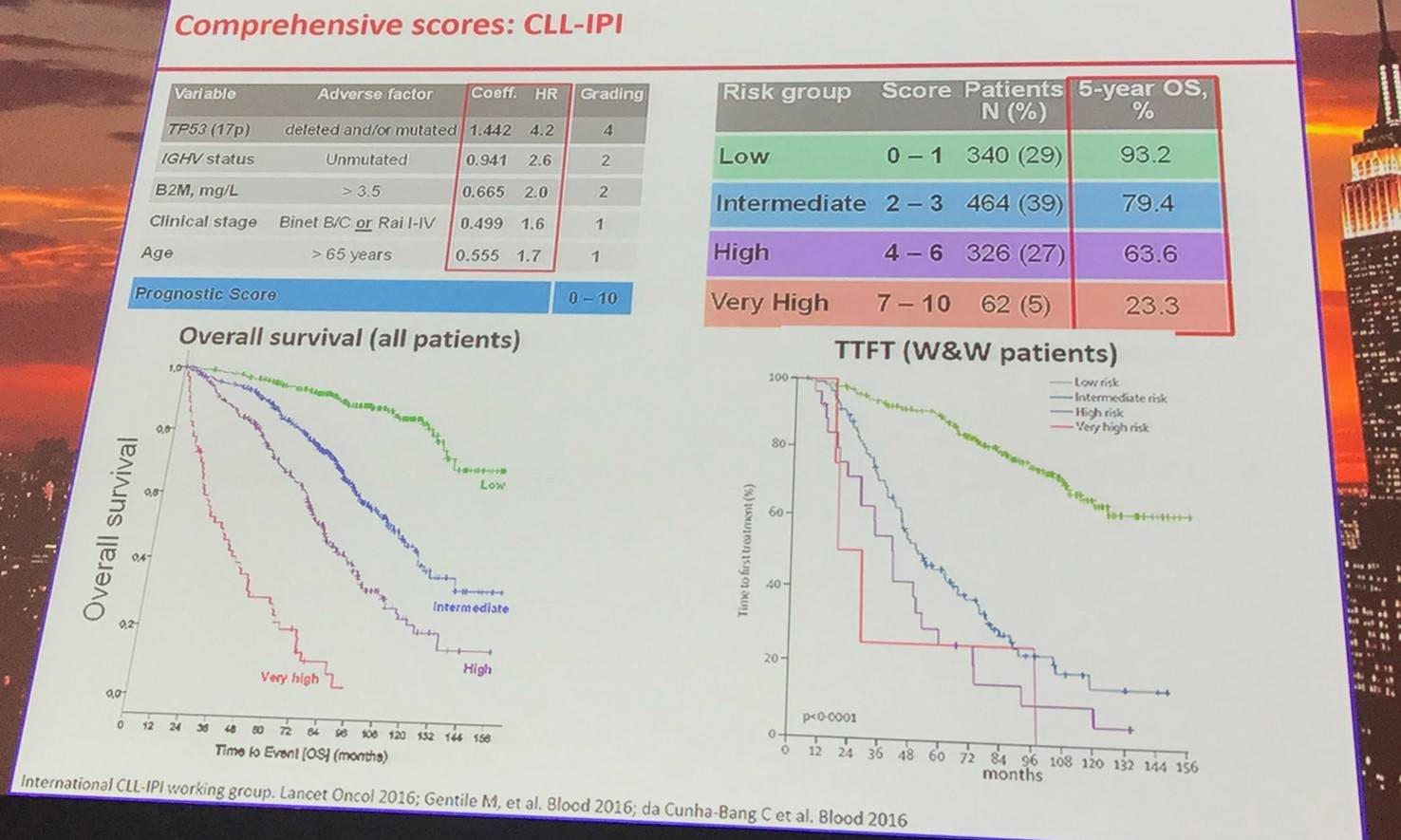
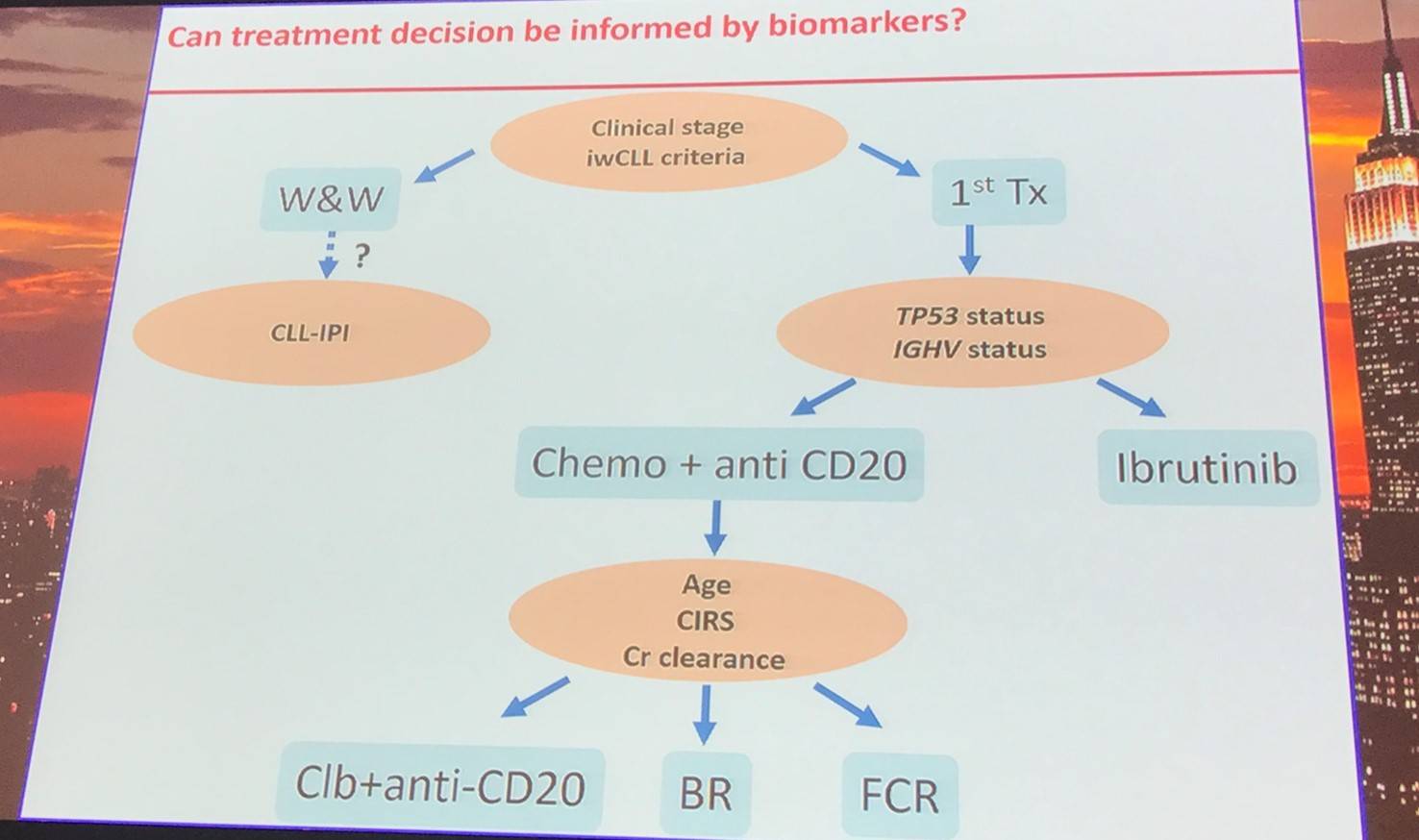
Clinical stage and iwCLL criteria assessment will determine if the disease in asymptomatic or symptomatic. Those with asymptomatic disease should be considered for patient counseling, the frequency of follow-up should be carefully evaluated, and patients should be identified for early intervention trials. For symptomatic patients, the most appropriate therapy should be selected.
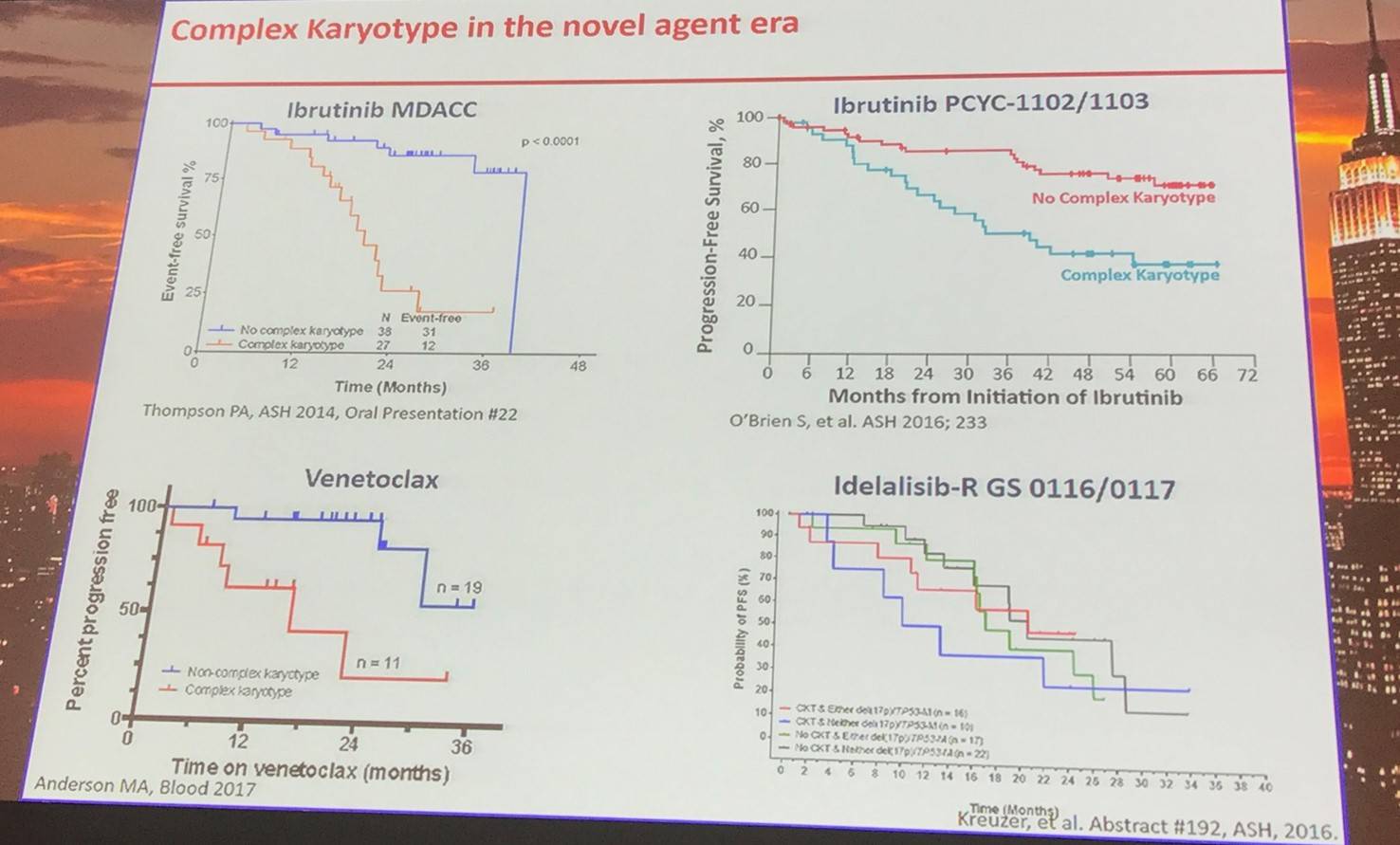
Abnormalities in TP53 are common in CLL, with their frequency increasing through MBL, early stage CLL, CLL requiring treatment, refractory CLL, and Richter transformation. Of TP53 abnormalities, 10% are deletion only, 20% are mutation only, and 70% are mutation and deletion.
The survival outcomes of R/R patients by chromosomal abnormality identified by FISH after treatment with ibrutinib have been previously reported (O’Brien et al. ASH 2016):
|
Genetic abnormality |
Median PFS |
5-year PFS |
Median OS |
5-year OS |
|---|---|---|---|---|
|
Del17p (n=34) |
26 months |
19% |
57 months |
32% |
|
Del11q (n=28) |
55 months |
33% |
NR |
61% |
|
Trisomy 12 (n=5) |
NR |
80% |
NR |
80% |
|
Del13q (n=13) |
NR |
91% |
NR |
91% |
|
No abnormality (n=16) |
NR |
66% |
NR |
83% |
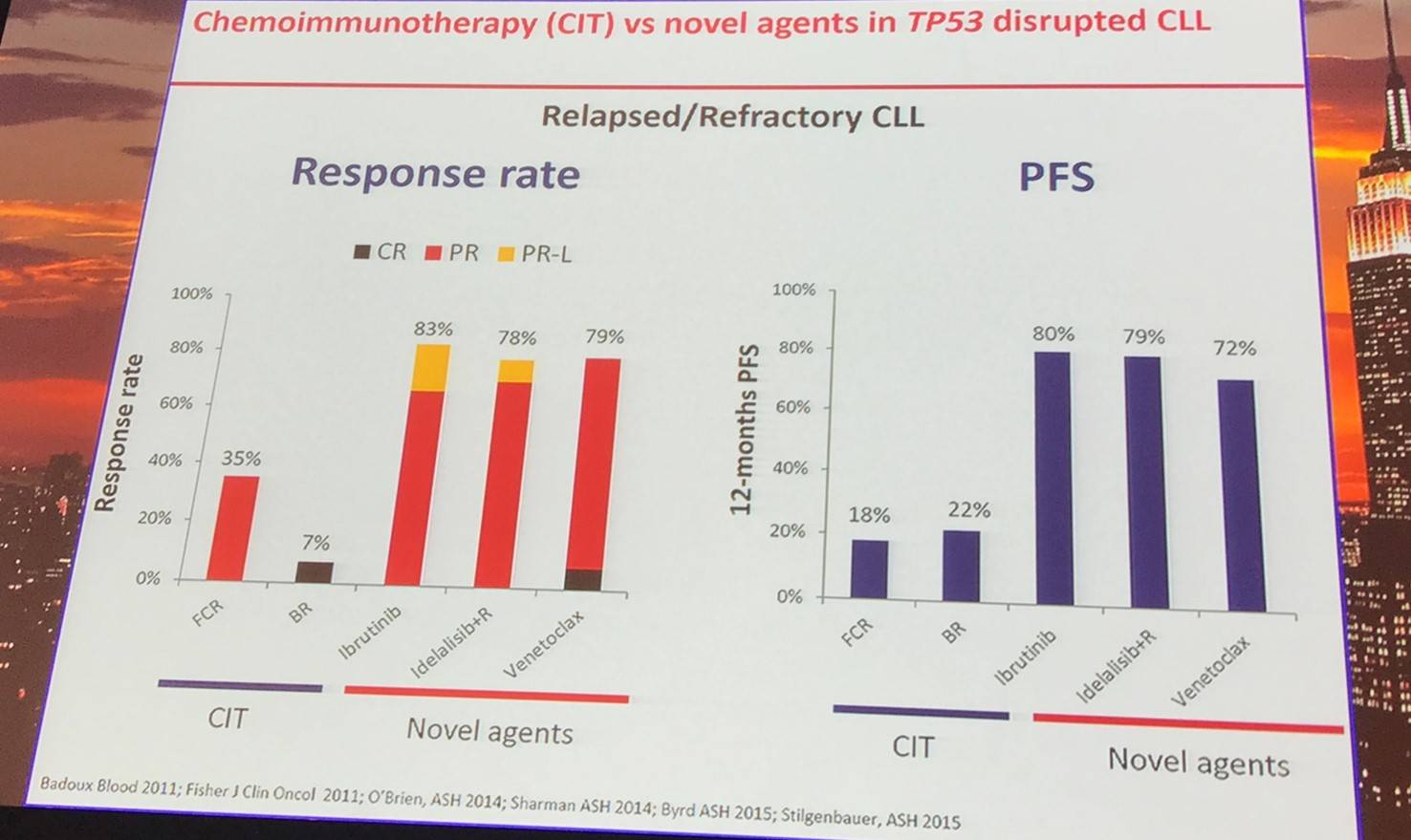
Molecular mechanisms of resistance to ibrutinib include:
- BTK C481S mutation
- PLCG2 mutation (several; gain of function)
- Absent in ibrutinib naïve CLL
- Most of CLL showing acquired resistance
- Enriched in CLL with 17p deletion
The survival outcomes in R/R patients by IGHV mutational status treated with ibrutinib have also been reported (O’Brien et al. ASH 2016):
|
Genetic abnormality |
Median PFS |
5-year PFS |
Median OS |
5-year OS |
|---|---|---|---|---|
|
Mutated IGHV (n=16) |
63 months |
53% |
63 months |
66% |
|
Unmutated IGHV (n=79) |
43 months |
38% |
NR |
55% |
CLL prognostic markers:
|
|
Validated prognostic biomarkers |
Other prognostic biomarkers |
|---|---|---|
|
Host factors |
Age |
Sex, etc. |
|
Disease markers |
Stage, LDT |
|
|
Antigen expression |
CD49d |
CD38, ZAP-70 |
|
Serology |
Β2M |
TK, LDH, sCD23 |
|
Genetics |
Del17p, TP53 mutation |
Del11q22, del13q14, trisomy 12, NOTCH1 mutation, SFRB1 mutation |
|
Biology markers |
IGHV-sequence |
BCR-structure |
References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

