All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
KEYNOTE-087: 5-year follow-up data of pembrolizumab in R/R classic Hodgkin lymphoma
Pembrolizumab, an inhibitor of programmed death 1 (PD-1), has been approved by both the U.S. Food and Drug Administration (FDA) and the European Medicine Agency for the treatment of patients with relapsed or refractory (R/R) classic Hodgkin Lymphoma (cHL). These approvals were based on the KEYNOTE-013 (NCT01953692) and the KEYNOTE-087 (NCT02453594) studies; the Lymphoma Hub previously reported key findings from the 2-year follow-up study of KEYNOTE-087. Below, we summarize a recent publication by Armand et al.1 on the 5-year follow-up results of pembrolizumab from the KEYNOTE-087 trial.
Study design1
KEYNOTE-087 is a multi-center, single-arm, non-randomized phase II study evaluating of pembrolizumab in adult patients with R/R cHL who had progressive disease (PD) after the most recent therapy or had not responded to a recent autologous stem cell transplant (ASCT).
Patients with cHL received 200 mg of pembrolizumab intravenously every 3 weeks for 2 years.
Patients were divided into the 3 cohorts:
- Cohort 1: with PD after ASCT and subsequent brentuximab vedotin (BV);
- Cohort 2: with PD after salvage chemotherapy and BV, ineligible for ASCT; and
- Cohort 3: with PD after ASCT without subsequent BV.
Patients achieving complete response (CR) who discontinued treatment and subsequently experienced PD were eligible for a second course of pembrolizumab. CR and PD were monitored by using computed tomography (every 12 weeks) and positron emission tomography (at Weeks 12 and 24).
The primary endpoints were objective response rate (ORR) by blinded independent central review (BICR) and safety.
Results
Efficacy1
Overall, 210 patients were included in the study.
- The median follow-up was 63.7 months.
- ORR per International Working Group revised response criteria for malignant lymphomas 2007 criteria by BICR for the overall population is shown in Figure 1.
Figure 1. Objective response rate per the IWG 2007 criteria by BICR*
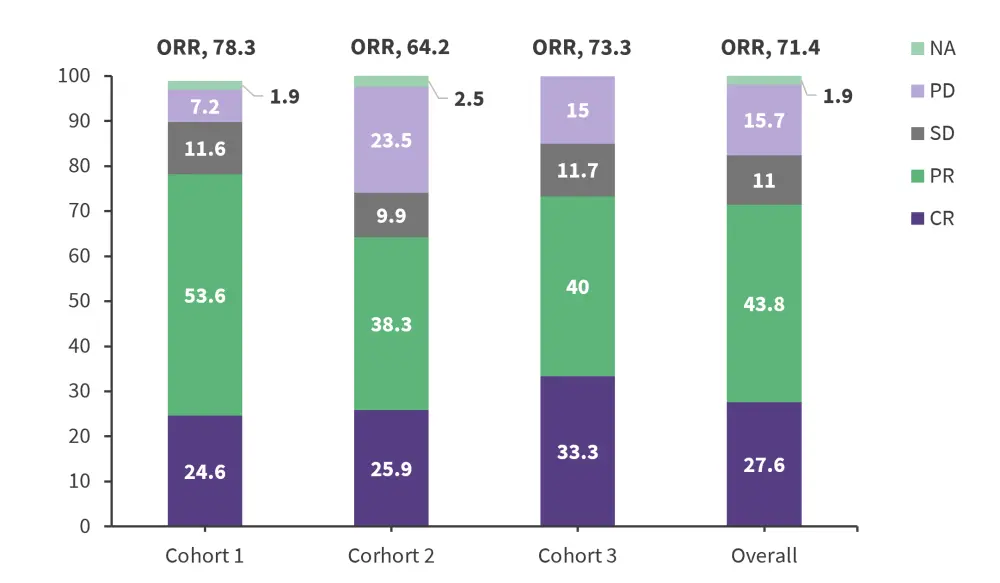
BICR, blinded independent central review; CR, complete response; IWG 2007, international working group revised response criteria for malignant lymphomas; NA, not assessed, ORR, objective response rate; PD, progressive disease; PR, partial response; SD stable disease.
*Adapted from Armand, et al.1
Median overall survival (OS) was not reached and 5-year OS was 70.7% for the overall population. Median DOR and PFS are shown in Figure 2.
Figure 2. Median DOR and PFS of overall population per IWG 2007 criteria*
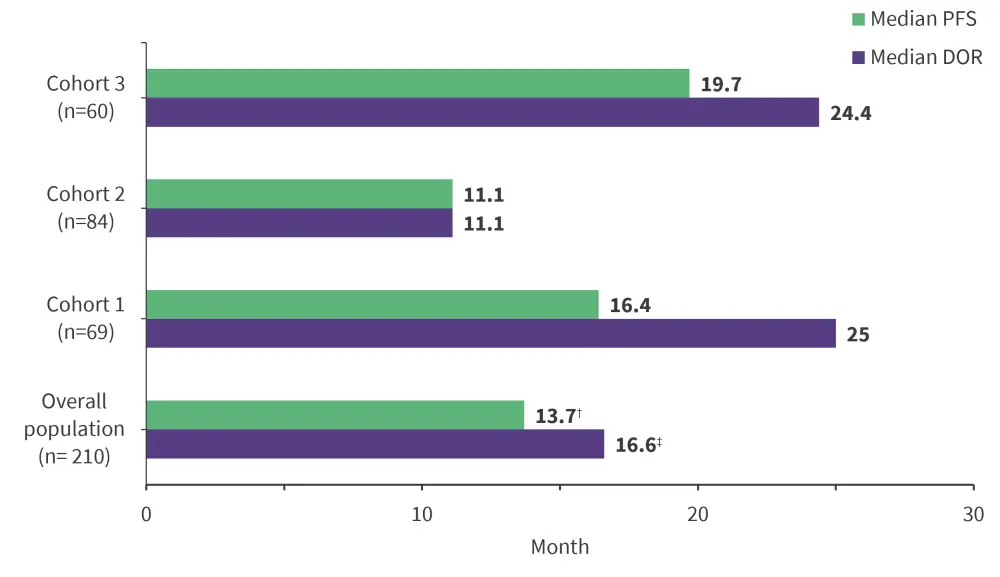
† 95% confidence interval (CI), 11.1-19.4.
‡95% CI, 11.8-27.11.
DOR, duration of response; PFS, progression-free survival.
*Adapted from Armand, et al.1
Efficacy by best objective response1
The 5-year OS rate in the overall population was 82.8%, with 10 patients received ASCT. Table 1 summarizes the efficacy responses in patients achieving CR.
Table 1. Median DOR and PFS in patients that achieved CR*
|
ASCT, autologous stem cell transplant; DOR, duration of response; PFS, progression-free survival, NR, not reached. |
||
|
Patients |
Median DOR |
Median PFS |
|---|---|---|
|
Overall population (n = 58) |
NR |
44.3% |
|
Patients who received ASCT (n = 10) |
13.6 months |
36.9 months |
|
Patients who did not receive ASCT (n = 48) |
NR |
56.5 months |
- Ninety-two patients across Cohorts 1 (n = 37), Cohort 2 (n = 21), and Cohort 3 (n = 24) achieved PR.
- Median DOR was 11.1 months
- Median PFS was 13.8 months, 3-year PFS was 10.3%
- The median OS was NR and 5-year OS rate was 75.5%
- Twenty-three patients achieved stable disease
- Median PFS was 8.3 months and 3-year PFS rate was 5.3%
- The median OS was NR and 5-year OS was 53.7%
- Thirty-three patients had disease progression
- Median PFS was 2.8 months
- Median OS was 62.9 months and 5-year OS was 50.06%
Efficacy in patients who received a second course of treatment1
Twenty patients received a second course of pembrolizumab across Cohort 1 (n = 10), Cohort 2 (n = 7), and Cohort 3 (n = 3) and 19 patients were evaluable.
The median duration of initial response before second-course treatment was 27.2 months.
- ORR was 73.7% (CR and PR of 36.8% each).
- Cohort 1: 77.8%
- Cohort 2: 85.7%
- Cohort 3: 33.3%
- Median DOR was 15.2 months.
- Among patients who experienced a CR with a second course of pembrolizumab (n = 7),
- Median DOR was 21.8 months
- Median PFS was 17.2 months and 2-year PFS was 38.1%
- The median OS was NR and 2-year and 5-year OS rates were 94.1 and 87.4, respectively
- Two patients received ASCT
Safety1
In the overall population, 97.6% of patients reported an adverse event (AE) and treatment-related AEs were reported in 72.9% of patients (12.9% were Grade 3/4). The most common treatment-related AEs were hypothyroidism, pyrexia, fatigue, and rash (Table 2). Fourteen patients (6.7%) discontinued the study because of treatment-related AEs. There were no treatment-related deaths.
Table 2. Treatment related adverse events*
|
†Reported in >10% of patients. |
||
| Treatment related adverse events, % | Overall population (N = 210) | |
|---|---|---|
| Any Grade† | Grade 3 or 4‡ | |
| Hypothyroidism | 14.3 | 0.0 |
| Pyrexia | 11.4 | 0.0 |
| Fatigue | 11.0 | 0.0 |
| Rash | 11.0 | 0.0 |
| Diarrhea | 8.1 | 1.0 |
| Headache | 7.6 | 0.0 |
| Nausea | 7.1 | 0.0 |
| Arthralgia | 6.2 | 0.0 |
| Cough | 6.2 | 0.0 |
| Pruritus | 6.2 | 0.0 |
| Infusion-related reaction | 5.2 | 0.0 |
| Neutropenia | 5.2 | 2.4 |
| Pericarditis | 1.0 | 1.00 |
Among the 20 patients who received second-course pembrolizumab treatment, 19 (95.0%) experienced a treatment-related AE with the most common AE were fatigue (20.0%), diarrhea, muscle spasm and rash (15% each). Seven Grade 3 treatment-related AEs were experienced in six patients. No patients discontinued the second course pembrolizumab or died of treatment-related AEs.
Conclusion
These data demonstrate that pembrolizumab continues to improve outcomes over a period of 5 years, without any new safety concerns in patients with R/R cHL who are ineligible, or who have progressed after ASCT.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



