All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Liso-cel as a second-line treatment for patients with R/R LBCL not intended for HSCT
Introduction
Only 50% of patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL), in whom first-line treatment did not yield a response, are eligible for high-dose chemotherapy and hematopoietic stem cell transplantation (HSCT). This highlights an unmet need in patients not intended for HSCT, given the historically poor prognosis and limited treatment options in this population.
Lisocabtagene maraleucel (liso-cel) is an autologous, CD19-directed, defined composition, 4-1BB chimeric antigen receptor (CAR) T-cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells. The Lymphoma Hub has previously reported the phase II TRANSFORM study results, recently published in The Lancet,1 which demonstrated the superiority of liso-cel over standard of care as second-line therapy for patients with R/R LBCL eligible for HSCT.
During the European Hematology Association (EHA) 2022 Congress, Nilanjan Ghosh presented the complimentary results of the phase II PILOT study evaluating the efficacy and safety of liso-cel in patients with R/R LBCL not intended for HSCT.2 Below we summarize the results, which have also recently been published in The Lancet Oncology.3
Study design
This is a multicenter, open-label, phase II study conducted in 18 clinical sites within the US. Eligible patients were:
- ≥18 years of age with R/R LBCL who had experienced disease progression after frontline chemotherapy, consisting of an anthracycline and CD20 targeted agent;
- not deemed eligible for transplant by their treating healthcare professional, based on age and comorbidities; and
- met one or more of the six transplant non-intended criteria as follows: age ≥70 years, Eastern Cooperative Oncology Group Performance Status of 2, diffusing capacity for carbon monoxide ≤60%, left ventricular ejection fraction <50%, creatinine clearance <60 mL/min, or alanine aminotransferase/aspartate aminotransferase >2 × the upper limit of normal.
Patients received lymphodepletion with cyclophosphamide (300 mg/m2) and fludarabine (30 mg/m2), followed 2–7 days later by liso-cel infusion at a dose of 100 × 106 CAR+ T cells (Figure 1).
Figure 1. Treatment schedule for patients not intended for HSCT infused with Liso-cel*
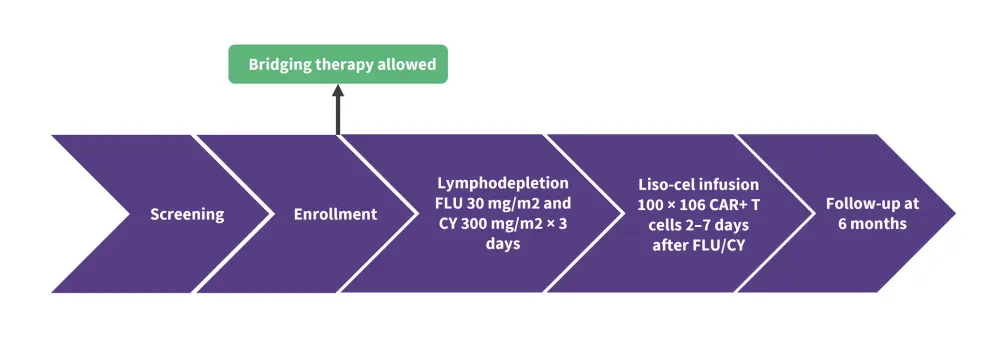
*Adapted from Ghosh.1
CY, cyclophosphamide; FLU, fludarabine; liso-cel, lisocabtagene maraleucel.
The primary endpoint was overall response rate determined by an independent review committee. Secondary endpoints included duration of response (DOR), complete response (CR) assessed by an independent review committee, progression-free survival (PFS), event-free survival (EFS), overall survival, adverse events, and laboratory abnormalities.
Results
Baseline characteristics
In total, 61 patients were treated with Liso-cel (Table 1).
Table 1. Selected baseline characteristics*
|
CR, complete response; DLBCL, diffuse large B-cell lymphoma; Fl3b, follicular lymphoma Grade 3b; HGBCL, high grade B-cell lymphoma; LBCL, large B cell lymphoma; LDC, lymphodepleting chemotherapy; LDH, lactate dehydrogenase; NOS, not otherwise specified; PD, progressive disease; PR, partial response; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHP, rituximab, cyclophosphamide, doxorubicin, and prednisone; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; R-ICE, rituximab, ifosfamide, carboplatin, and etoposide; SD, stable disease; tFL, transformed follicular lymphoma; ULN, upper limit of normal. |
|
|
Characteristic, % (unless otherwise stated) |
Liso-cel treated patients (n = 61) |
|---|---|
|
Median age (range), years |
74 (53–84) |
|
Pre-LDC LDH |
|
|
<500/≥500 unit/L |
82/18 |
|
≤ULN/>ULN |
39/61 |
|
Histology |
|
|
DLBCL NOS |
54 |
|
tFL |
15 |
|
HGBCL with DLBCL histology |
30 |
|
FL3b |
2 |
|
Double or triple hit |
33 |
|
Bone marrow involvement at baseline |
11 |
|
Relapsed or refractory |
|
|
Refractory |
54 |
|
Relapsed ≤12 months |
21 |
|
Relapsed >12 months |
25 |
|
Best response to first-line therapy for LBCL |
|
|
CR/PR |
46/25 |
|
SD/PD |
8/21 |
|
First-line treatment for LBCL |
|
|
Radiotherapy |
11 |
|
Systemic treatment |
100 |
|
R-CHOP |
84 |
|
R-EPOCH |
16 |
|
R-ICE, R-CHP, and/or other |
13 |
Efficacy
- Overall response rate was 80% for all responders (partial response + CR) and CR rate was 54% (Figure 2).
- Median DOR for patients achieving CR was 21.7 months, compared with 12.1 months for all responders.
- Median PFS and EFS was 9.0 and 7.2 months, respectively, and both were 22.6 months in patients who achieved CR.
- Median overall survival was not reached.
Figure 2. Response rates in patients with R/R with LBCL treated with Liso-cel *
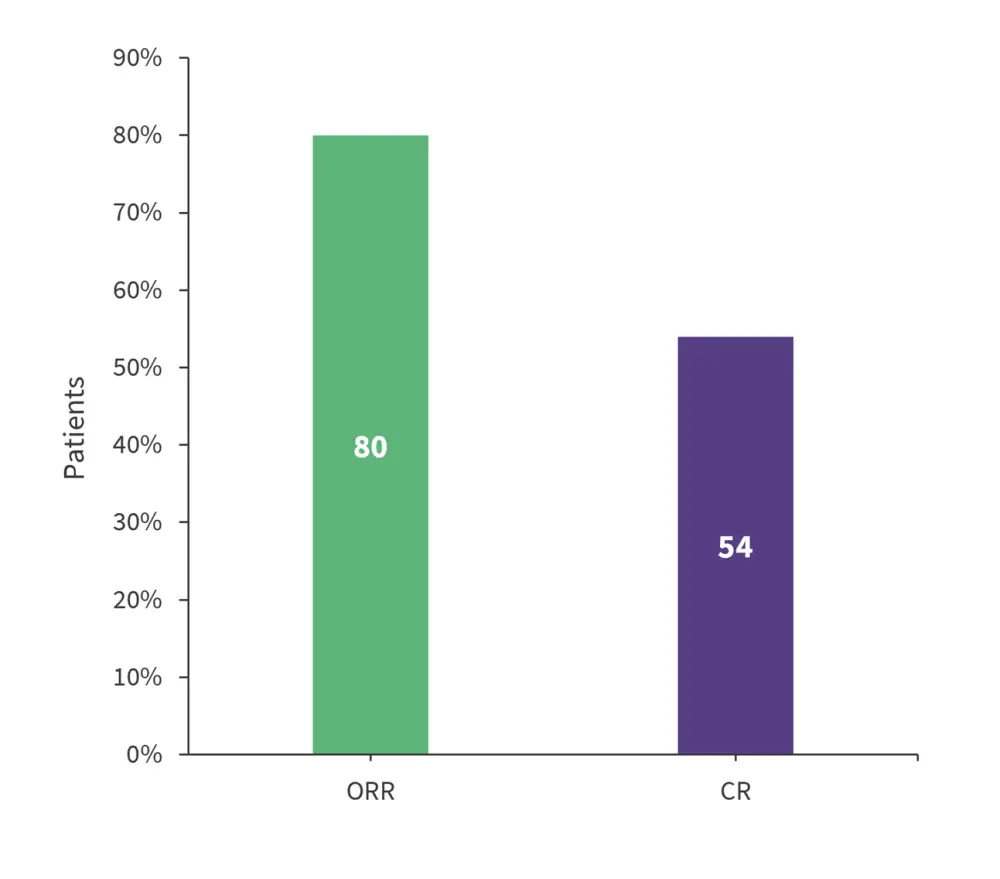
CR, complete response; ORR, overall response rate.
*Adapted from Ghosh.1
Safety
The most frequent treatment-emergent adverse events across all grades were neutropenia (51%), fatigue (39%), and cytokine-release syndrome (CRS; 38%). The most common Grade ≥3 treatment-emergent adverse events were neutropenia (48%), leukopenia (21%), and thrombocytopenia (20%; Figure 3).
Overall, CRS incidence was 38% (n = 23) and neurological events (NEs) occurred in 31% of patients (n = 19). There was a low incidence of Grade 3 CRS and NEs (n = 1 and n = 3, respectively) and no Grade 4/5 CRS and NEs were reported. Only one of 16 patients required more than one dose of tocilizumab to manage CRS. There were no treatment-related deaths reported and there was no prophylactic use of corticosteroids and vasopressors.
Figure 3. TEAE’s for ≥15% patients not intended for HSCT who were treated with Liso-cel*
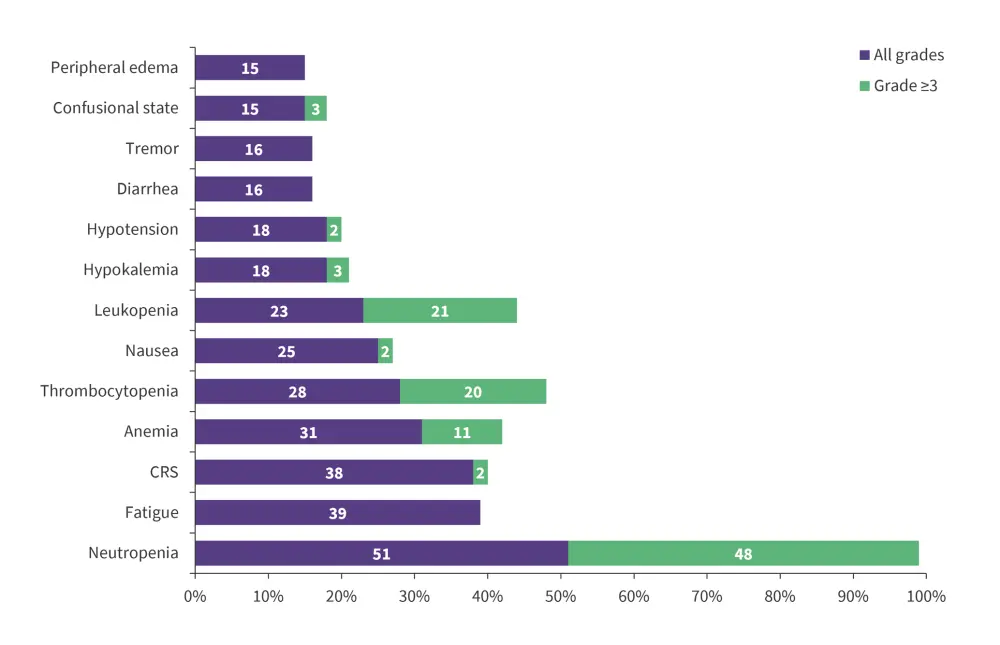
CRS, cytokine release syndrome.
*Adapted from Ghosh.1
Conclusion
The results demonstrated the effectiveness of liso-cel as a second-line treatment for patients with R/R LBCL who are not intended for HSCT, with a median DOR of 12.1 months and overall response rate of 80%. These findings complement the TRANSFORM phase II study results, which reported liso-cel was superior to standard of care for those intended for HSCT. Given there were no new safety signals, a low incidence of Grade 3 CRS and NEs, and no Grade 4/5 CRS and NEs, liso-cel could offer a potential new treatment for patients with R/R LBCL not intended for HSCT.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

