All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Lisocabtagene maraleucel (liso-cel), is an investigational, CD19-directed, defined composition, 4-1BB chimeric antigen receptor (CAR) T-cell product administered at equal doses of CD8+ and CD4+ CAR T cells, and it is currently under evaluation in several subtypes of relapsed or refractory (R/R) lymphoma.
TRANSCEND-CLL-004 (NCT03331198) is a phase I/II trial investigating the safety and efficacy of liso-cel as monotherapy or in combination with ibrutinib in patients with R/R chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). Initial phase I results of the combination cohort were presented by William Wierda during the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.1
TRANSCEND-NHL-001 (NCT02631044) is a phase I seamless design study evaluating the safety and efficacy of liso-cel in B-cell non-Hodgkin lymphoma. During ASH 2020, Maria Lia Palomba presented the initial results from the R/R mantle cell lymphoma (MCL) cohort receiving liso-cel.2 Liso-cel has been recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with R/R diffuse large B-cell lymphoma (DLBCL), Grade 3B follicular lymphoma (FL), or primary mediastinal large B-cell lymphoma (PMBCL), based on favorable results from TRANSCEND-NHL-001. European Medicines Agency approval is currently being sought.
Here, we are pleased to summarize these two talks.
Update from the TRANSCEND-CLL-004 trial1
In this study, patients with R/R CLL/SLL received lymphodepleting chemotherapy followed by liso-cel infusion. Results from the phase I combination cohort (outlined in red in Figure 1) were presented. The results of the phase I monotherapy arm (n = 23) were previously summarized here.
Figure 1. TRANSCEND-CLL-004 study design1
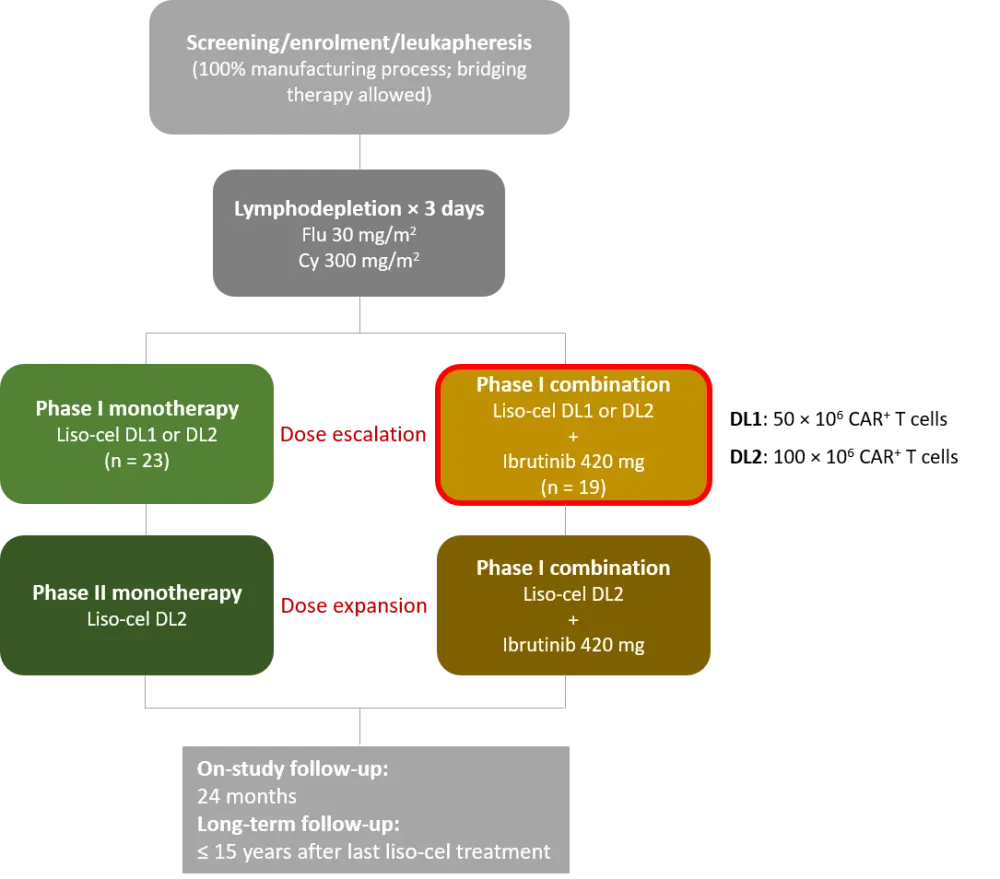
CAR, chimeric antigen receptor; Cy, cyclophosphamide; DL, dose level; Flu, fludarabine; liso-cel, lisocabtagene maraleucel.
The phase I combination cohort included patients:
- With R/R CLL/SLL who progressed on ibrutinib at the time of enrolment; or
- With high-risk features and received ibrutinib for ≥ 6 months but achieved less than a complete response (CR); or
- With ibrutinib-resistant mutations including BTK or PLCɣ2 mutations; or
- Who had prior ibrutinib exposure without contraindications to reinitiating ibrutinib
Patients continued or restarted ibrutinib up to a target dose of 420 mg at enrolment, for up to 90 days following liso-cel infusion, or longer in case of clinical benefit. Bridging therapy was allowed.
Primary objectives were safety and determination of recommended dose for the dose expansion part of the study. Dose-limiting toxicities (DLTs) were evaluated in the first 28 days after liso-cel infusion.
Results
Table 1 provides baseline characteristics among dose levels. Most patients (95%) had high-risk disease, were heavily pretreated, and were all R/R to ibrutinib.
Table 1. Patient characteristics1
|
BTKi, Bruton tyrosine kinase inhibitor; DL, dose level; R/R, relapsed or refractory; Ven, venetoclax. |
|||
|
Characteristic |
Combination cohort (DL1/DL2 + ibrutinib; |
DL1 + ibrutinib |
DL2 + ibrutinib |
|---|---|---|---|
|
Median age, years (range) |
61 (50–77) |
58 (50–70) |
61 (51–77) |
|
Median time since diagnosis, months (range) |
121 (21–252) |
84 (31–176) |
127 (21–252) |
|
Bulky disease ≥ 5 cm, n (%) |
6 (32) |
0 (0) |
6 (40) |
|
Received bridging therapy, n (%) |
8 (42) |
2 (50) |
6 (40) |
|
Stage, n (%) |
|
|
|
|
High-risk feature (any), n (%) |
18 (95) |
4 (100) |
14 (93) |
|
Median prior lines, n (range) |
4.0 (1–10) |
4.5 (1–5) |
3.0 (2–10) |
Safety
Liso-cel plus ibrutinib combination was well tolerated with no DLTs observed.
- Any grade cytokine release syndrome (CRS) occurred in 14 patients (74%) with a median time to onset of 6.5 days (range, 1–13) and a median duration of 6 days (range, 3–13)
- Any grade neurological events (NEs) occurred in 6 patients (32%) with a median time to onset of 8 days (range, 5–12) and a median duration of 6.5 days (range, 1–8)
- Tocilizumab and/or corticosteroids were used to manage these events
- No Grade 4 CRS or NEs were reported
Table 2 summarizes Grade ≥ 3 adverse events (AEs) at both dose levels. No Grade 5 AEs were reported. Dose reductions or treatment discontinuation due to ibrutinib-related treatment-emergent AEs (TEAEs) were uncommon.
Table 2. Safety outcomes1
|
AE, adverse events; CRS, cytokine release syndrome; DL, dose level; NE, neurological event; TEAE, treatment-emergent AE. |
|||
|
AE, n (%) |
Combination cohort (DL1/DL2 + ibrutinib; |
DL1 + ibrutinib |
DL2 + ibrutinib |
|---|---|---|---|
|
Grade 3/4 TEAEs |
18 (95) |
4 (100) |
14 (93) |
|
Grade 3 CRS |
1 (5) |
1 (25) |
0 (0) |
|
Grade 3 NEs |
3 (16) |
0 (0) |
3 (20) |
|
Grade 3/4 ibrutinib-related TEAEs* |
7 (37) |
2 (50) |
5 (33) |
|
Ibrutinib dose reduction due to TEAE |
2 (11) |
0 (0) |
2 (13) |
|
Ibrutinib discontinuation due to TEAE |
4 (21) |
1 (25) |
3 (20) |
Response
Best overall response and rates of undetectable minimal residual disease (uMRD; sensitivity of ≤ 10–4) are shown in Figure 2.
Figure 2. Best overall response and uMRD at 10-month follow-up1
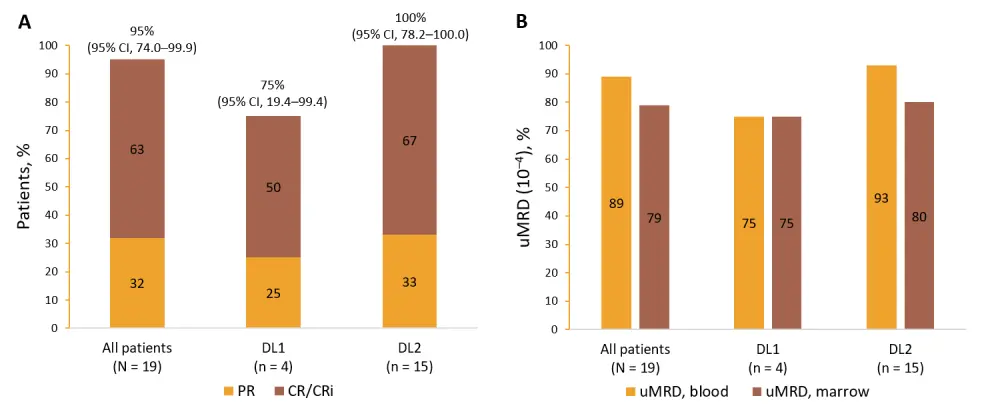
A Best overall response; B uMRD at any time point.
CI, confidence interval; CR, complete response; CRi, CR with incomplete hematologic recovery; DL, dose level; PR, partial response; uMRD, undetectable minimal residual disease (≤ 10-4).
Most patients remained in remission at 10-month follow-up. Patients who responded achieved an early response by Day 30 after liso-cel infusion.
The two liso-cel dose levels did not affect CAR+ T-cell expansion differently, while median peak CAR+ T-cell expansion occurred by Day 11 in the combination cohort.
Conclusion
In summary, these initial findings suggest a favorable tolerability with low rates of Grade 3 CRS or NEs. This heavily pretreated patient cohort with R/R CLL/SLL achieved rapid and high response rates and uMRD when liso-cel was combined with ibrutinib. The safety profile was similar among dose levels, and DL2 (100 × 106 CAR+ T cells) was identified as the recommended dose for the expansion cohort. Further evaluation of the combination of liso-cel and ibrutinib is currently ongoing.
Combining CAR T-cell therapy with targeted drugs—can we improve efficacy in patients with R/R CLL?
Update from the TRANSCEND-NHL-001 trial2
The TRANSCEND-NHL-001 trial included two patient cohorts: (i) large B-cell lymphoma (LBCL), and (ii) MCL. In the LBCL cohort, 269 patients received liso-cel; study design and patient characteristics can be found here. This update focuses on the dose finding and expansion parts of the MCL cohort, where the safety and initial antitumor activity of liso-cel was assessed.
The MCL cohort included patients with MCL who had received ≥ 2 lines of therapy, and who had previous exposure to BTK inhibitors, alkylating agents, and an anti-CD20 agent. Prior stem cell transplantation and secondary central nervous system lymphoma were allowed. The primary endpoints included AEs, DLTs, and overall response rate (ORR).
A total of 32 patients (Table 3) treated with liso-cel in two dose levels (DLs) following a 3-day lymphodepletion (30 mg/m2 fludarabine and 300 mg/m2 cyclophosphamide for 3 days):
- DL1: 50 × 106 CAR+ T cells (n = 6)
- DL2: 100 × 106 CAR+ T cells (n = 26)
Table 3. Patient characteristics2
|
ECOG PS, Eastern Cooperative Oncology Group Performance Status; BTKi, Bruton tyrosine kinase inhibitor; CNS, central nervous system; HSCT, hematopoietic stem cell transplantation; MCL, mantle cell lymphoma. |
|
|
Characteristic |
MCL cohort (N = 32) |
|---|---|
|
Age |
|
|
ECOG PS, n (%) |
|
|
Blastoid morphology, n (%) |
13 (41) |
|
Ki67 ≥ 30%, n (%) |
23 (72) |
|
TP53 mutations, n (%) |
7 (22) |
|
Secondary CNS lymphoma, n (%) |
1 (3) |
|
Bridging therapy, n (%) |
17 (53) |
|
Prior therapies |
|
|
Median number (range) |
3 (1–7) |
|
HSCT, n (%) |
11 (34) |
|
BTKi, n (%) |
28 (88) |
|
Venetoclax, n (%) |
8 (25) |
Safety
All patients treated with liso-cel experienced at least one TEAE of any grade, and 84% experienced Grade ≥ 3 TEAEs (Table 4). Two patients who received DL2 experienced Grade 5 TEAEs which were considered related to liso-cel (n = 1, tumor lysis syndrome; n = 1, cryptococcal meningoencephalitis leading to death). Two DLTs were recorded, both at DL2, and included tumor lysis syndrome, and neutropenia/thrombocytopenia.
Table 4. Grade ≥ 3 AEs2
|
AE, adverse event; CRS, cytokine release syndrome; NE, neurological event; TEAE, treatment-emergent AE; TLS, tumor lysis syndrome. |
|
|
Grade ≥ 3 AEs, n (%) |
N = 32 |
|---|---|
|
TEAEs |
27 (84) |
|
CRS |
1 (3) |
|
NEs |
4 (12.5) |
|
Prolonged cytopenia* |
11 (34) |
|
Infections |
5 (16) |
|
TLS |
1 (3) |
Response rates
Median on-study follow-up was 5.9 months (range, 0.4–24.8), and median time to first CR or PR was 0.95 months (range, 0.9–2.0). Figure 3 presents best overall responses in all patients and those with high-risk features.
Figure 3. Best overall response2
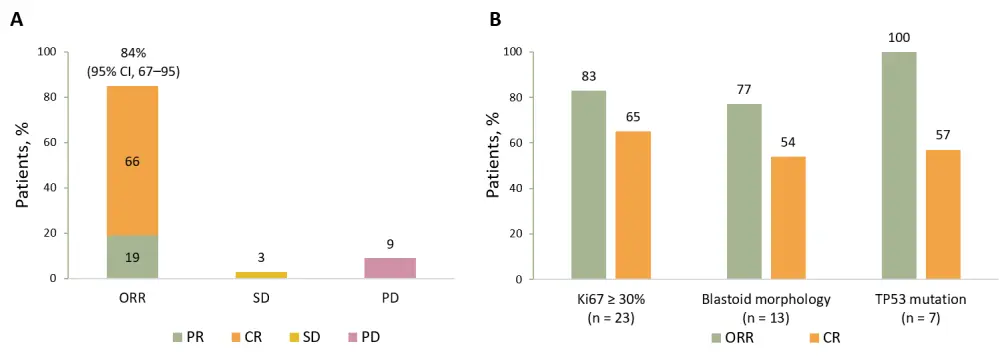
A Best overall response in all patients (N = 31); B Response rates in patients with high-risk features.
CI, confidence interval; CR, complete response; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Median duration of response was not reached at a median follow-up of 3.9 months. Six patients had durable responses over 1 year, and responses were mostly similar in patients with blastoid morphology and those resistant to ibrutinib.
The median time of maximal liso-cel expansion was achieved 10 days following infusion. Long-term liso-cel persistence at 1 year and 2 years was recorded in 67% and 33% of patients, respectively.
Conclusion
These findings indicate promising results in terms of safety and clinical activity of liso-cel in patients with R/R MCL. The rate of Grade ≥ 3 TEAEs was low, with Grade ≥ 3 CRS and NE rates at 3% and 12.5%, respectively. Response rates were encouraging, with an ORR of 84%, CR of 66%, and median duration of response was not reached. Enrolment in the MCL cohort is ongoing with DL2.
Related: The FDA recently granted approval to brexucabtagene autoleucel for the treatment of R/R MCL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 William Wierda
William Wierda