All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Long-term toxicities associated with ibrutinib in patients with CLL
Do you know... Which of the following cardiovascular events was associated with baseline hypertension in ibrutinib-treated patients with chronic lymphocytic leukemia at long-term follow-up?
The introduction of targeted therapies, such as Bruton’s kinase inhibitors, has significantly improved outcomes for patients with chronic lymphocytic leukemia (CLL). Ibrutinib was the first Bruton’s kinase inhibitor approved by the U.S. Food and Drug Administration (FDA) in 2014 for patients with previously treated CLL; approval was based on results from the phase III RESONATE trial (NCT01578707) in which ibrutinib demonstrated a higher efficacy compared with ofatumumab. In the phase III E1912 trial (NCT02048813), ibrutinib plus rituximab demonstrated a higher progression-free survival and overall survival (OS) in untreated CLL when compared with fludarabine, cyclophosphamide, and rituximab.1
Despite the clinical efficacy of ibrutinib in CLL, patients discontinue treatment due to common adverse events, such as new-onset hypertension and other cardiovascular toxicities. Here, we summarize an article published by Gordon et al.1 in Cancer on the long-term toxicities associated with ibrutinib in patients with CLL, with a focus on hypertension and cardiovascular effects.
Study design
The retrospective study included patients aged ≥18 years who were enrolled and treated across six different ibrutinib-based clinical trials from 2010 to 2017 at MD Anderson Cancer Center, Houston, US.
- The primary outcome was the development of new or worsening hypertension.
- New hypertension was defined as a systolic blood pressure (SBP) of ≥130 mg Hg and/or diastolic BP (DBP) of ≥80 mg Hg on two separate visits after starting ibrutinib.
- Worsening hypertension was defined as a second reading showing a ≥10 mg Hg increase in DBP above baseline.
- Severe hypertension was defined as the second reading of a SBP ≥140 mm Hg or DBP of ≥90 mm Hg.
- Very severe hypertension was defined as a second measurement of an SBP ≥160 mm Hg or DBP of ≥100 mm Hg.
- Secondary outcomes included the incidence of cardiovascular and renal complications associated with hypertension as well as the associations of baseline characteristics with new-incident cardiovascular events, event-free survival (EFS), and OS.
Results
A total of 300 patients were included, comprising patients who were predominantly male and Caucasian, had comorbidities, relapsed/refractory disease, and high-risk features. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
Characteristic, % (unless otherwise stated) |
(N = 300) |
|
Median age at enrollment (range), years |
65 (29–83) |
|
Sex |
|
|
Male |
70 |
|
Female |
30 |
|
Race |
|
|
Caucasian |
88 |
|
Black |
8 |
|
Obese (BMI ≥30 mg/m2) |
34 |
|
Tobacco use |
2.7 |
|
Comorbidities |
|
|
Coronary heart disease |
7.3 |
|
Hyperlipidemia |
43 |
|
Chronic kidney disease |
9.4 |
|
Congestive heart failure |
1.3 |
|
Obstructive sleep apnea |
10 |
|
Atrial fibrillation |
6.3 |
|
Hypertension |
69 |
|
Baseline SBP, mm Hg |
|
|
<100 |
2 |
|
100–119 |
22 |
|
120–129 |
22 |
|
130–139 |
24 |
|
140–179 |
30 |
|
≥180 |
0.7 |
|
Baseline DBP, mm Hg |
|
|
<70 |
30 |
|
70–79 |
35 |
|
80–89 |
29 |
|
90–119 |
5.7 |
|
Therapy |
|
|
Ibrutinib monotherapy |
52 |
|
Ibrutinib + rituximab |
44 |
|
Ibrutinib + BR |
4.7 |
|
R/R disease |
88 |
|
Rai stage |
|
|
0–1 |
56 |
|
2 |
10 |
|
3–4 |
18 |
|
FISH analysis |
|
|
Normal |
15 |
|
Deletion 11q |
18 |
|
Trisomy 12 |
9 |
|
Deletion 13q |
17 |
|
Deletion 17p |
17 |
|
TP53 mutation |
5 |
|
Mutated IGHV |
17 |
|
Unmutated IGHV |
53 |
|
Elevated B2 microglobulin |
46 |
|
Normal B2 microglobulin |
52 |
|
BMI, body mass index; BR, bendamustine rituximab; DBP, diastolic blood pressure; FISH, fluorescence in situ hybridization; IGHV, immunoglobulin heavy chain variable; R/R, relapsed/refractory; SBP, systolic blood pressure. |
|
Blood pressure changes and hypertension
At the 5-year follow-up, there was an increase in median SBP and DBP across the total cohort in both men and women, regardless of baseline hypertension, obesity, or cardiovascular morbidity. Of the 71 patients who had hypertension while on ibrutinib treatment, 38% retained hypertension after ibrutinib discontinuation and 11.2% developed new-onset and persistent hypertension after ibrutinib discontinuation.
The incidence of new hypertension for patients who did not have hypertension at baseline, worsening hypertension in patients with baseline hypertension, and severe or very severe hypertension 5 years after the start of ibrutinib treatment are summarized in Figure 1.
Figure 1. Incidence of new-onset, worsening, severe, and very severe hypertension at 5-year follow-up*
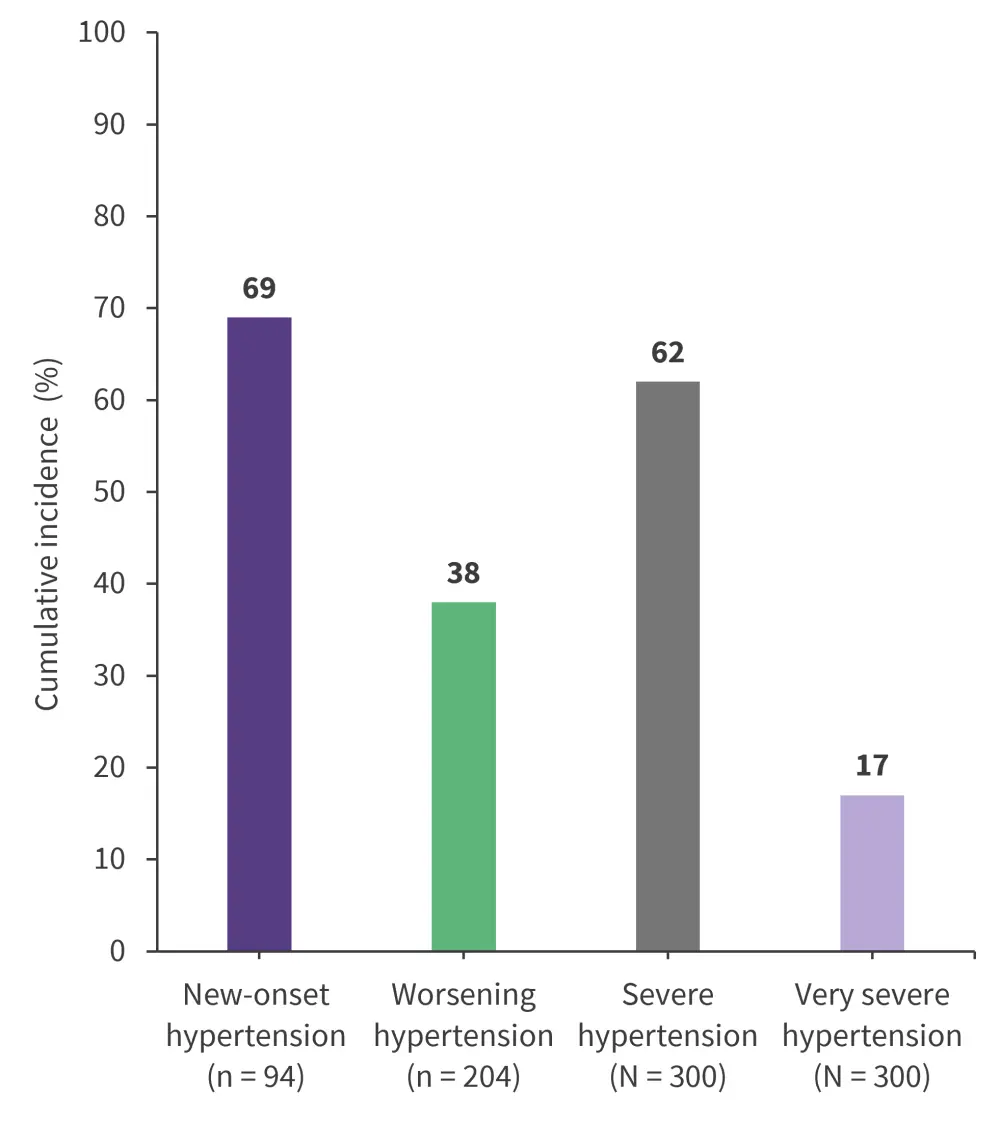
*Data from Gordon, et al.1
Association of hypertension and cardiovascular events
In the univariate analyses:
- Male sex, tobacco use, and chronic kidney disease were associated with the development of severe hypertension (p < 0.01, p = 0.02, and p = 0.04, respectively).
- Older age was significantly associated with very severe hypertension (p = 0.04).
- The combination of ibrutinib with bendamustine and rituximab was associated with both new onset and very severe hypertension compared with ibrutinib monotherapy (p < 0.01).
- Baseline hypertension was not associated with major adverse cardiovascular events, including new coronary artery disease, congestive heart failure, atrial fibrillation, stroke, cardiovascular death, EFS, or OS, but was significantly associated with ischemic stroke and intracranial hemorrhage in ibrutinib-treated patients with CLL (p = 0.013 and p = 0.03, respectively; Figure 2).
- Overall, 183 patients discontinued ibrutinib, 84 due to adverse events, 75 due to disease progression, and 24 due to other reasons.
- The most common adverse events leading to treatment discontinuation included non-cardiovascular toxicity in 13.3%, cardiovascular toxicity in 8.7%, and secondary cancers in 6%.
Figure 2. The association of baseline hypertension with cardiovascular events*
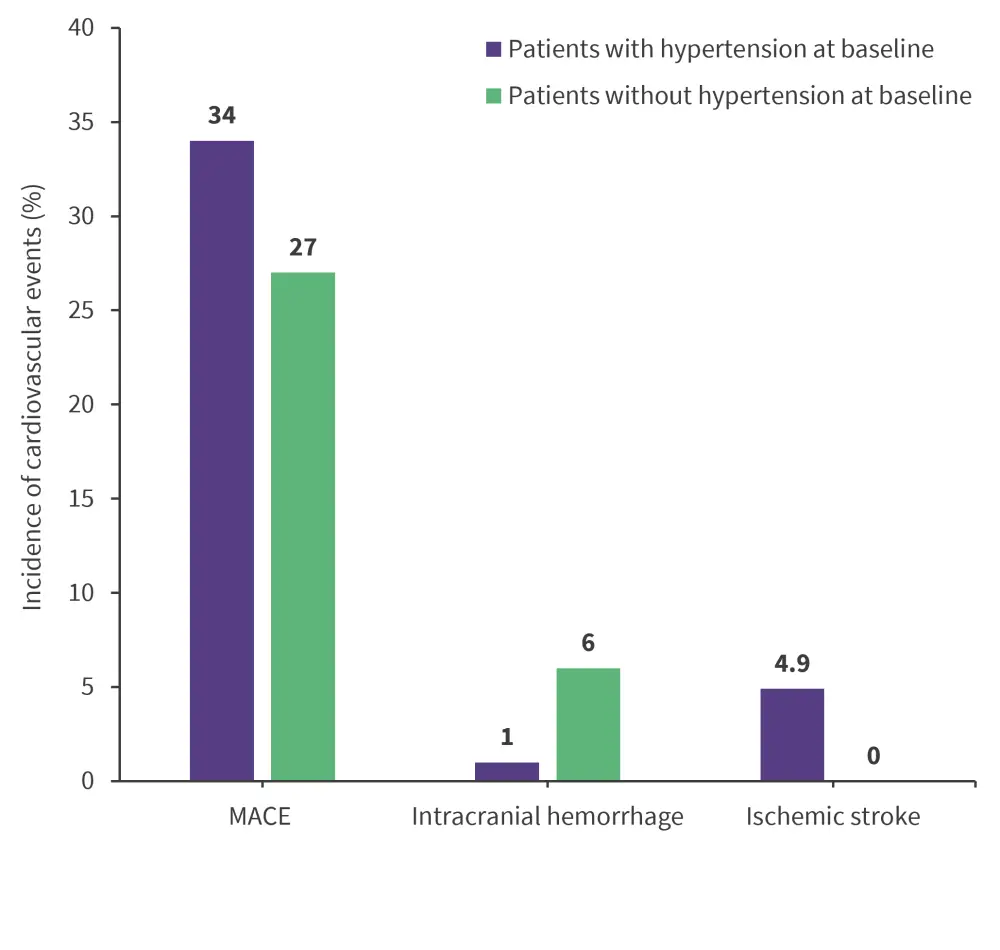
MACE, major adverse cardiovascular events.
*Data from Gordon, et al.1
At the 5-year follow-up, median OS was not reached in the total cohort with an estimated 5-year OS of 69.9%. The median EFS was 45.1 months, with an estimated 5-year OS of 39%. Two deaths occurred due to sudden cardiac death or arrest in male patients who had baseline hypertension and coronary heart disease.
Conclusion
Despite the retrospective nature, this study showed that hypertension is a common long-term effect of ibrutinib; however, it was shown to be manageable and reversible in patients with CLL. Hypertension was not associated with major adverse cardiovascular events or survival outcomes, and baseline characteristics associated with ibrutinib-related hypertension included:
- chronic kidney disease;
- older age;
- male sex; and
- tobacco use
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

