All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
MRD-guided treatment with ibrutinib + venetoclax: Updates from the FLAIR trial
Do you know... Based on the updates from the FLAIR trial presented at ASH 2025, which of the following statements is true?
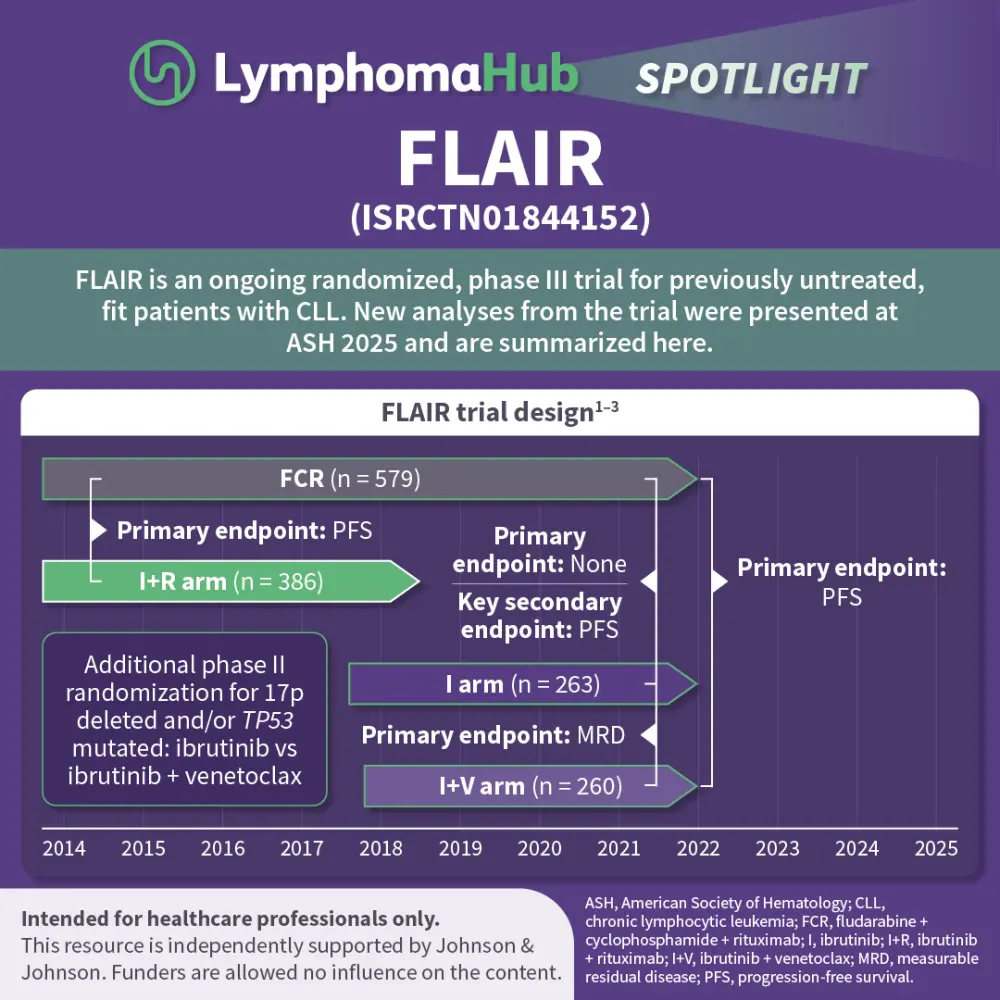
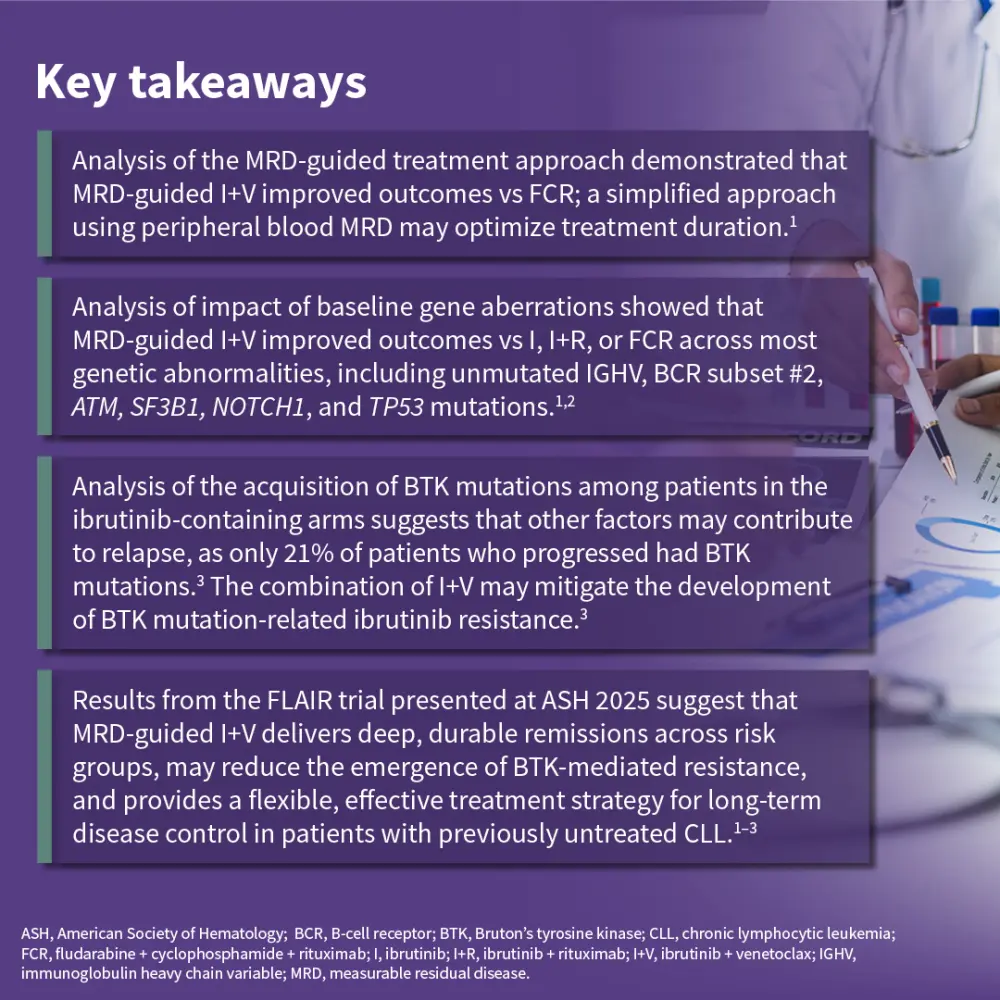
Results from the multicenter, open-label, randomized, phase III FLAIR trial (ISRCTN01844152), which were previously covered by the Lymphoma Hub, demonstrated that measurable residual disease (MRD)-guided treatment with ibrutinib + venetoclax improved outcomes vs ibrutinib alone or fludarabine + cyclophosphamide + rituximab (FCR) in previously untreated patients with chronic lymphocytic leukemia (CLL).1–3 Several updates from the FLAIR trial were presented by Hillmen1, Evans2, and Dalal3 during the 67th ASH Annual Meeting and Exposition 2025, December 6–9, 2025, Orlando, US.
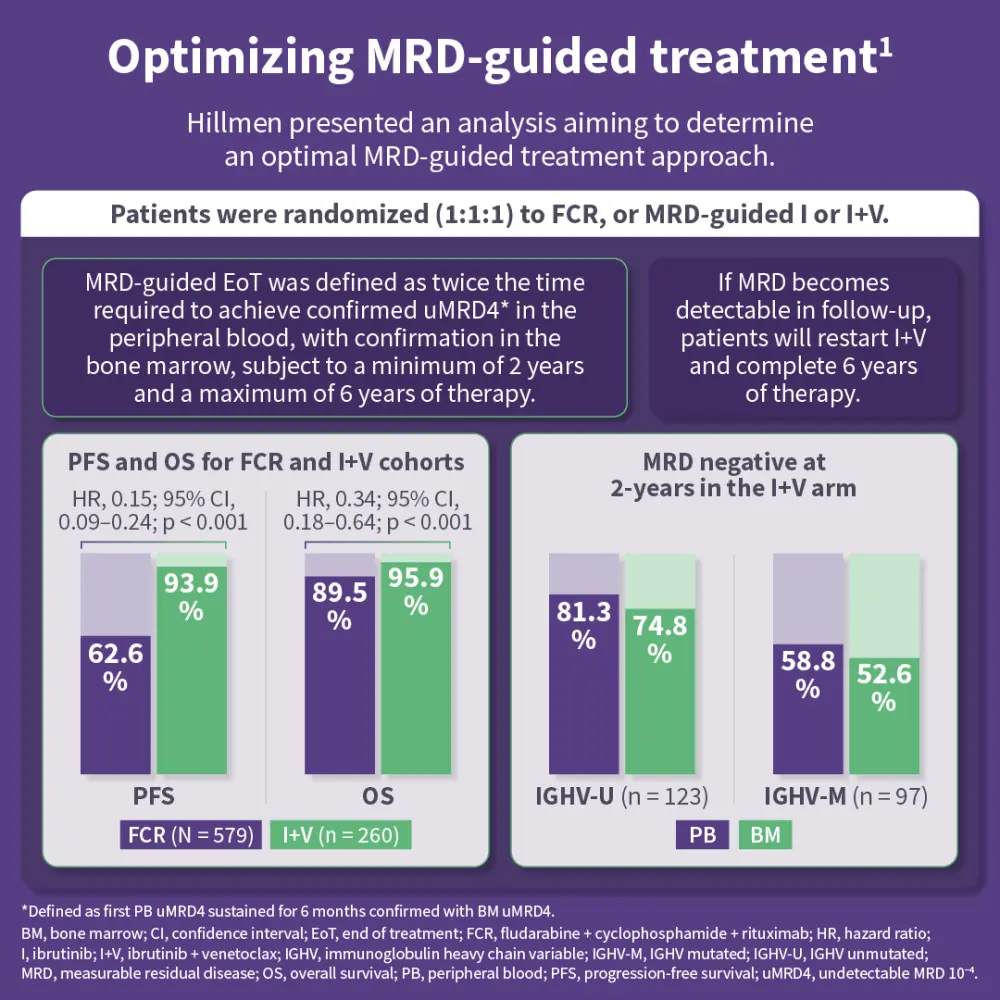
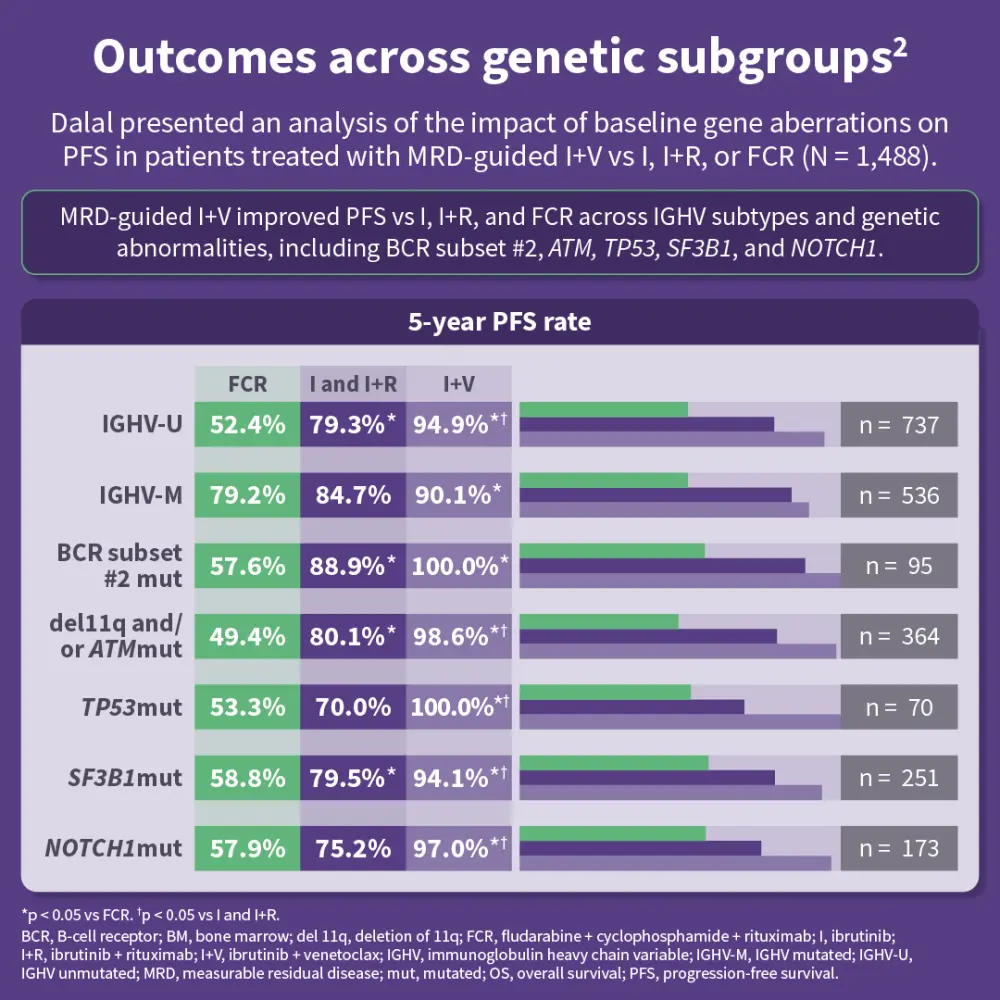
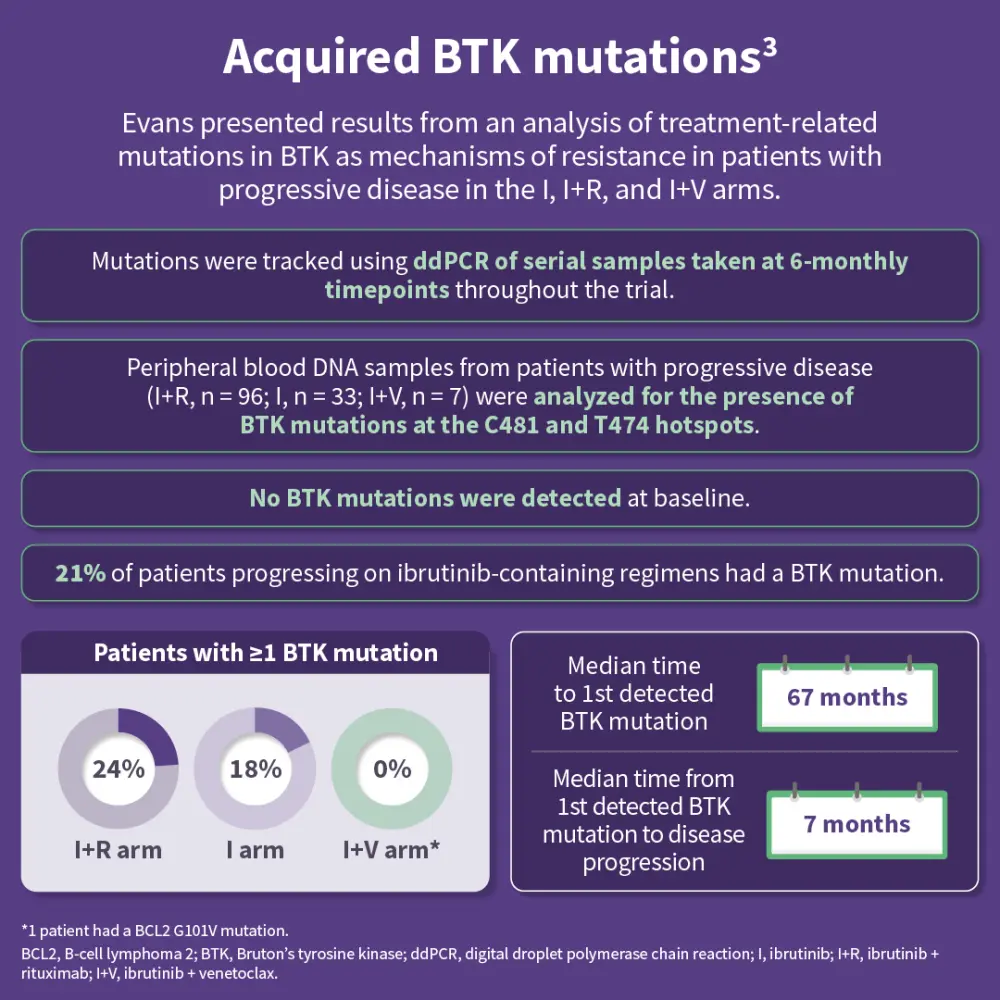
The FLAIR trial was initially designed to assess FCR vs ibrutinib + rituximab and, due to a lack of benefit from adding rituximab, was adapted in 2017 to include an ibrutinib monotherapy arm and an MRD-guided ibrutinib + venetoclax arm.1 Hillmen presented an analysis aiming to determine an optimal MRD-guided treatment approach.1 Dalal presented an analysis of the impact of baseline gene aberrations on outcomes in the FLAIR trial.2 Finally, Evans shared an analysis of treatment-related mutations in Bruton’s tyrosine kinase (BTK) as mechanisms of resistance in patients with progressive disease in the ibrutinib-containing arms.3
Results from these analyses suggest that MRD-guided ibrutinib + venetoclax is associated with deep and durable remissions across risk groups, may reduce the emergence of BTK-mediated resistance, and represents an effective treatment strategy for patients with previously untreated CLL.1–3

This educational resource is independently supported by Johnson & Johnson. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




