All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Novel targeted therapies for R/R marginal zone lymphoma
It has been challenging to define an optimal therapeutic approach for relapsed patients with marginal zone lymphoma (MZL) due to disease heterogeneity, especially in the advanced, refractory setting. The current standard of care is localized treatment and/or chemoimmunotherapy, but eventually, disease relapses, and there is a need for subsequent alternative therapies and an optimized sequencing strategy. Moreover, MZL management recommendations are often based on follicular lymphoma results, trials with a mixed population with indolent B-cell lymphomas, or retrospective reviews.1,2
It is known that B-cell receptor-mediated signaling plays a significant role in MZL pathogenesis, and this is the reason why Bruton’s tyrosine kinase (BTK) inhibitors were tested for the treatment of patients with MZL. Ibrutinib, a first-generation BTK inhibitor, demonstrated an overall response rate (ORR) of 48% and in 2017 became the first targeted therapy approved in the US for relapsed MZL.2
New targeted therapies are being explored for relapsed/refractory (R/R) MZL, including parsaclisib and zanubrutinib. Below you will find a summary of the initial efficacy and safety data of both targeted agents, presented recently at the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.
Zanubrutinib: A next-generation BTK inhibitor1
Zanubrutinib is a next-generation, irreversible BTK inhibitor optimized for a higher specificity to improve efficacy and safety. In an early phase study, single-agent zanubrutinib was effective in R/R MZL with an ORR of 80%, which led to the phase II study MAGNOLIA (NCT03846427). The following data were presented by Stephen Opat from Monash Health, Melbourne, AU.
The MAGNOLIA trial enrolled a total of 68 patients with R/R MZL who had received ≥ 1 prior treatment line, including an anti-CD20 therapy. The primary endpoint was ORR, with zanubrutinib 160 mg administered twice a day, evaluated by an independent review committee based on the Lugano classification. However, for this interim analysis, only the investigator-reported results were presented.
Results
Enrolled patients had a median age of 70 years, with 60.3% aged ≥ 65 years, and the vast majority had a performance status of 0–1. Overall, the median lines of prior systemic therapy were 2 (range, 1–6), and one-third of patients had refractory disease. After a median follow-up of 10.7 months, 44 patients (64.7%) remained on study treatment, while 24 discontinued due to progressive disease (28%), adverse events (3%), or investigator/patient’s choice (4.5%).
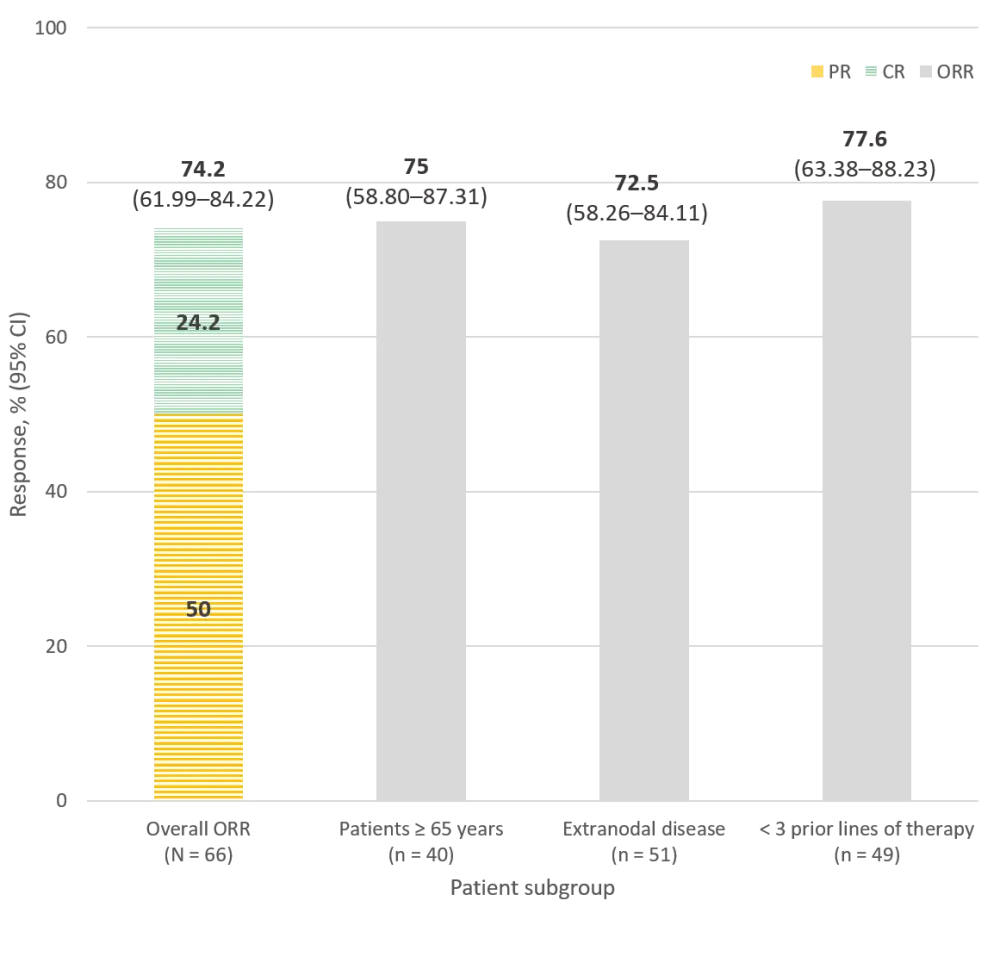
Looking at the evaluable population (N = 66), most patients had some response to zanubrutinib, achieving an ORR (complete and partial responses) of 74.2%, plus 15.2% of patients experienced a stabilization of their disease. Moreover, the investigators reported consistent response rates across different subgroups, even when comparing several prior treatments (Figure 1). Preliminary data on duration of response (DOR) and survival outcomes show a 6-month DOR rate of 79% and a progression-free survival (PFS) rate of 80%, which reduces to 67% at 9 months.
Figure 1. Overall response rates in patients with R/R MZL treated with zanubrutinib, reported by the investigators1
Almost all patients treated with zanubrutinib experienced at least one treatment-emergent adverse event (TEAE), of which 38.2% were Grade ≥3. Fortunatelythese TEAEs rarely led to study discontinuation or death. The most frequently reported Grade ≥3 TEAEs were infection (13.2), neutropenia (10.3%), diarrhea, pyrexia, thrombocytopenia, anemia, pneumonia, and second primary malignancy (all with a 2.9% incidence). Overall, the investigators reported a favorable safety profile of zanubrutinib.
Conclusion
Zanubrutinib demonstrates to be a safe and highly active next-generation BTK inhibitor, even in the heavily pretreated and elderly population recruited in the MAGNOLIA trial. The data is not mature yet, but a significant clinical benefit was observed in 89% of patients.
To further characterize the favorable tolerability profile of zanubrutinib, a new phase II study is recruiting patients with B-cell lymphoma, including MZL, intolerant to previous treatment with ibrutinib and/or acalabrutinib to monitor the recurrence and change in the severity of TEAEs of interest (NCT04116437).
Parsaclisib: A novel PI3Kδ inhibitor2
Clinical activity has been previously reported with phosphoinositide 3-kinase (PI3K) inhibitors in relapsed or refractory B-cell lymphomas, including MZL, with ORRs ranging 39–70%. Parsaclisib is a next-generation PI3Kδ inhibitor with a characteristic structure that enables a more potent and highly selective target union than other members of the same drug family, leading to a reduction of transaminitis incidence.
The CITADEL-204 trial (NCT03144674) is a phase II study evaluating parsaclisib in patients with nodal, extranodal, or splenic R/R MZL who received at least one prior therapy (including ≥ 1 anti-CD20 antibody). Tycel J. Phillips from the Rogel Cancer Center, Ann Arbor, US, presented preliminary data on efficacy and safety.
Initially, this trial assessed several treatment schedules with parsaclisib and included patients previously exposed to ibrutinib. However, the ibrutinib-exposed arm was closed due to slow recruitment; after the release of new emergent data from other parsaclisib trials, BTK inhibitor-naïve patients’ preferred regimen was defined as parsaclisib 20 mg once daily for 8 weeks, followed by 2.5 mg daily (instead of 20 mg weekly), continuously.
Results
A total of 100 patients were enrolled, including 72 patients in the cohort with the preferred dose of parsaclisib. Overall, 72% of patients were older than 65 years, the median time since MZL diagnosis was 4.6 years (range, 0.1–20.1), and performance status was good (0–1) in 95%. Patients had received a median of two prior lines of therapy, including:
- Chemotherapy (72%)
- Surgery or surgical procedures (19%)
- Radiation (11%)
- Hematopoietic stem cell transplantation (4%)
After a median follow-up of 16.7 months and a median of 11.4 months on treatment with parsaclisib, 42% of patients remained on treatment, with 28 patients receiving the continuous daily regime. The primary (ORR) and secondary efficacy endpoints are shown in Figure 2 and Table 1.
Figure 2. Overall response rates in patients with R/R MZL treated with parsaclisib2
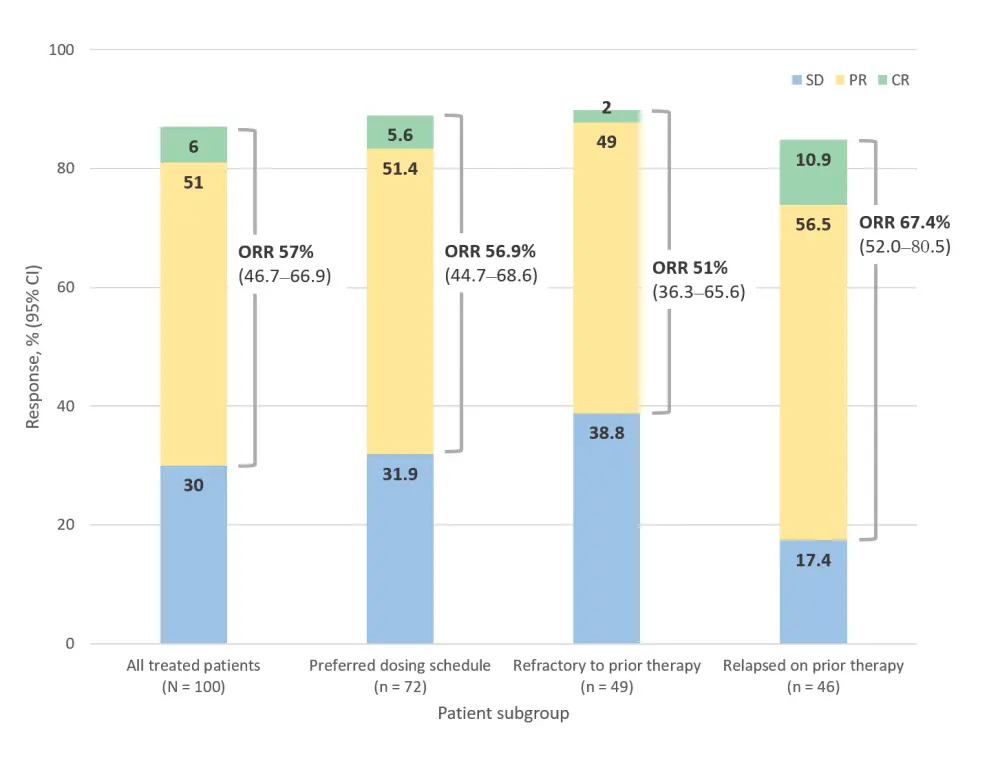
Table 1. Secondary efficacy endpoints in patients with R/R MZL treated with parsaclisib2
|
DOR, duration of response; NE, not evaluable; NR, not reported; PFS, progression-free survival. |
|
|
Secondary efficacy endpoint |
Response |
|---|---|
|
Median time to first response, weeks |
8.1 |
|
Median DOR, months (95% CI) |
12.0 (9.3–NE) |
|
Median DOR with the preferred dosing schedule |
NR (8.1–NE) |
|
Median PFS, months (95% CI) |
19.4 (13.7–NE) |
|
Median PFS with the preferred dosing schedule |
NR (11.0–NE) |
To date, the most frequent reasons for parsaclisib discontinuation were progressive disease (26%) and adverse events (25%), the latter being even more frequent with the parsaclisib preferred dose (32% of patients). Patients reporting Grade ≥3 TEAEs (59%) mainly experienced diarrhea, neutropenia, and anemia. Two patients died due to treatment-related sepsis and respiratory distress.
Conclusion
The first-in-class PI3Kδ inhibitor, parsaclisib, achieved rapid and durable responses in patients with R/R MZL, with comparable efficacy in patients with nodal, extranodal, and splenic MZL. Its safety profile was generally well tolerated and significantly improved compared with the phase I trial results (NCT02018861).3
Parsaclisib is also effective in treating patients with R/R follicular lymphoma (CITADEL-203, NCT03126019), and together with these results, a placebo-controlled phase III trial will start soon recruiting patients with R/R FL and MZL to receive parsaclisib combined with rituximab or obinutuzumab (CITADEL-302, NCT04796922).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

