All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Phase I dose expansion trial of brentuximab vedotin and lenalidomide in patients with R/R DLBCL
Most patients with diffuse large B-cell lymphoma (DLBCL) respond well to frontline therapy, but about 30% may be refractory to or relapse after first-line chemotherapy. A small proportion of these patients respond to salvage regimens, autologous stem cell transplantation (ASCT), or chimeric antigen receptor T-cell therapy (CAR-T). However, new treatment options are needed for ineligible patients or for those in which currently available treatments do not yield a response.
Brentuximab vedotin (BV), an anti-CD30 antibody conjugate and lenalidomide, an immunomodulatory agent, have demonstrated promising efficacy as single agents in patients with relapsed/refractory (R/R) DLBCL. BV and lenalidomide also have safety profiles that are generally favorable and that, together with their unique mechanisms of action and low likelihood of antagonistic effects, led to the study of this combination in the R/R setting of DLBCL. Here we summarize the key findings from a recent investigator-initiated phase I/dose expansion trial (NCT02086604) evaluating the combination of BV + lenalidomide in patients with R/R DLBCL, published by Ward, et al.1 in Blood.
Study design
This was a phase I, multicenter, open label study of BV + lenalidomide in patients with R/R DLBCL. Eligible patients were aged ≥18 years, had histologically confirmed R/R de novo or transformed DLBCL following at least one prior therapy, and had an Eastern Co-operative Oncology Group (ECOG) performance status of ≤2 with adequate organ function. Eligible patients also had measurable disease, CD30 immunohistochemical staining on the most recent biopsy and had received or were ineligible for ASCT.
The treatment cycle was 21 days with a maximum of 16 cycles (Figure 1). Dose limiting toxicities were defined as any of the following occurring during Cycle 1:
- Grade 4 neutropenia lasting >7 days
- Grade 4 infection with Grade 3–4 neutropenia
- Grade 4 thrombocytopenia associated with life-threatening bleeding or requiring >1 transfusion
- Treatment delays >14 days due to hematologic toxicity
- Grade 3–4 non-hematologic toxicities
Figure 1. Treatment schema*
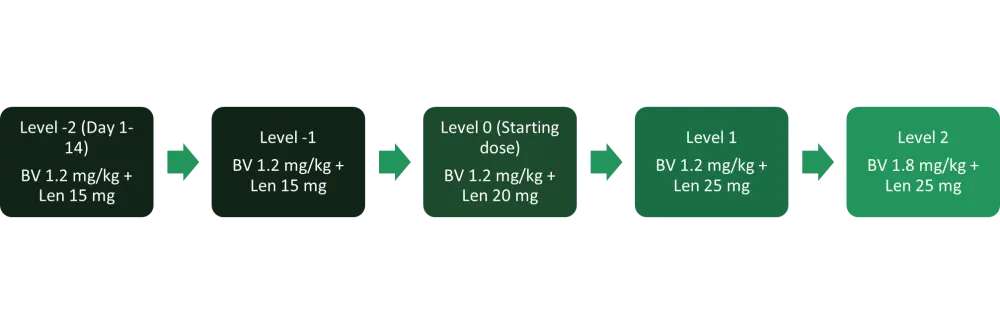
BV, brentuximab vedotin; IV, intravenously; Len, lenalidomide.
*Adapted from Ward, et al.1
The primary outcomes were adverse events (AEs) and maximum tolerated dose (MTD) of BV + lenalidomide. Secondary outcomes included:
- Overall response rate (ORR) (complete response [CR] + partial response [PR]).
- Responses were assessed according to the 2007 Revised Response Criteria for Malignant Lymphoma
- Duration of response
- Progression free survival
Results
Baseline characteristics
A total of 37 out of 38 enrolled patients were evaluable for toxicity and efficacy. The median age was 65 years (range, 51–79 years) and the median time from initial diagnosis was 14 months (range, 4–138 months). The baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
ASCT, autologous stem cell transplantation; ECOG, Eastern Co-operative Oncology Group performance status; GCB, germinal center B-cell; IHC, immunohistochemistry; LDH, lactate dehydrogenase; IPI, International Prognostic Index. |
|
|
Characteristics, % |
BV + lenalidomide (n = 37) |
|---|---|
|
Baseline ECOG Performance Status |
|
|
0–1 |
76 |
|
2 |
21 |
|
IPI score at study entry |
|
|
0 |
8 |
|
1 |
8 |
|
2 |
46 |
|
3 |
22 |
|
4 |
11 |
|
5 |
5 |
|
Transformed disease |
24 |
|
Elevated LDH |
54 |
|
Cell of origin by IHC |
|
|
GCB |
54 |
|
Non-GCB |
46 |
|
Stage at screening |
|
|
Stage I–II |
24 |
|
Stage III–IV |
76 |
|
Primary refractory |
46 |
|
Refractory to most recent treatment |
54 |
|
Patients with prior rituximab exposure |
100 |
|
Bulky disease (≥7.5 cm) |
35 |
|
Prior stem cell transplant |
27 |
|
Reasons of ASCT ineligibility |
|
|
Age |
33 |
|
Co-morbidities |
3 |
|
Inadequate response to salvage |
64 |
|
Median prior therapies (range) |
3 (1–6) |
MDT and safety
The MDT was dose level (DL) 0, BV 1.2 mg/kg + lenalidomide 20 mg, with 28 of the 37 patients initiating therapy at this dose level. Six patients were treated at DL1 (BV 1.2 mg/kg + lenalidomide 25 mg) with no dose limiting toxicities (DLTs), though most patients in this group required lenalidomide dose reductions within the first few cycles due to cytopenias. Three patients were treated at DL2 (BV 1.8 mg/kg + lenalidomide 25 mg) with two DLTs and both treatment delays >14 days were due to prolonged Grade 3-4 neutropenia.
In total, 94% percent of patients experienced at least one Grade 3–4 AE. The most common Grade 1–2 AEs occurring in >10% of patients and Grade 3–4 AEs are summarized in Table 2.
At least one dose reduction was required in 73% of patients, with dose reductions of lenalidomide and BV in 55% and 41% of patients, respectively. Although the lenalidomide dose was reduced to 5 mg, three patients discontinued due to toxicity (one patient each due to neutropenia, thrombocytopenia, or both). One patient died due to a potential treatment-related AE occurring during Cycle 1 along with profound cytopenia and rapidly progressing disease.
Overall, 84% of patients required granulocyte colony-stimulating factor (G-CSF), 55% of whom initiated G-CSF prophylactically with Cycle 1 and 45% of whom initiated after experiencing neutropenia or febrile neutropenia. Twenty three of the 28 patients (82%) treated at the MTD received G-CSF.
Table 2. Grade 1-2 AEs in >10% of patients and Grade 3-4 AEs*
|
AEs, adverse events. |
||
|
AEs, % |
Grade 1–2 |
Grade 3–4 |
|---|---|---|
|
Blood and lymphatic system |
||
|
Leukopenia |
73 |
32 |
|
Thrombocytopenia |
70 |
27 |
|
Anemia |
70 |
24 |
|
Neutropenia |
65 |
59 |
|
Lymphocytopenia |
59 |
43 |
|
Metabolic |
||
|
Hypocalcemia |
62 |
3 |
|
Hypoalbuminemia |
59 |
8 |
|
Hypokalemia |
54 |
27 |
|
Neurological |
||
|
Peripheral sensory neuropathy |
41 |
3 |
|
Skin |
||
|
Maculo-papular rash |
46 |
24 |
|
Others |
|
|
|
Fatigue |
41 |
8 |
|
Fever |
35 |
¾ |
Efficacy
- The median time to best response was 1.4 months (range, 0.6–8 months) and the median duration of response was 13.1 months.
- The ORR was 57%, with 35% of patients achieving CR and 22% achieving PR.
- The median time to follow-up was 14.3 months, with median progression free survival and OS of 10.2 and 14.3 months, respectively.
- A total of 29 patients died, 25 of whom died from progressive disease.
- Twenty patients had germinal center B-cell (GCB) and 17 had non-GCB as the cell of origin based on Han’s criteria.
- Patients in the GCB cohort demonstrated an ORR of 50% compared to 65% in the non-GCB cohort. CRs were achieved in six patients and PRs in four patients in the GCB cohort and in seven and four patients, respectively, in the non-GCB cohort.
- Positive CD30 (CD30+) expression ≥1% on neoplastic cells was observed in 41%, while 59% were CD30 negative.
- Response rate was highest in patients with CD30+ DLBCL (73%) including 40% of patients achieving CRs (Table 2).
Table 2. Responses based on CD30 status and DLBCL subtype*
|
CR, complete response; GCB, germinal center B-cell; PR, partial response; PD, progressive disease; ORR, overall response rate; SD, stable disease. |
||||
|
|
CD30 status/Han’s criteria |
|||
|---|---|---|---|---|
|
Responses, % |
CD30+/GCB |
CD30-/GCB |
CD30+/non-GCB |
CD30+/non-GCB |
|
CR |
50 |
17 |
29 |
50 |
|
PR |
37.5 |
8 |
29 |
20 |
|
SD |
12.5 |
33 |
0 |
0 |
|
PD |
0 |
42 |
43 |
30 |
|
ORR (CR+PR) |
87.5 |
25 |
57 |
70 |
Conclusion
This phase I trial demonstrated that BV + lenalidomide was fairly well tolerated in patients with R/R DLBCL, with most patients having neutropenia and thrombocytopenia as DLTs and requiring G-CSF. Responses were seen in both GCB and non-GCB, with more responses seen in patients with CD30+ DLBCL. The study established that that the MTD was 1.2 mg/kg of BV and 20 mg of lenalidomide due to dose reductions and DLTs at the DL1 and DL2 levels, respectively. The findings are limited by the small sample size and the lack of a comparator arm. However, the results of this phase I/dose escalation study show that BV + lenalidomide may be a promising salvage regimen for patients who are not eligible for or have relapsed following ASCT or CAR-T. An international, randomized phase III study (NCT04404283) of BV or placebo in combination with lenalidomide and rituximab has been initiated (ECHELON-3) to further explore the efficacy of this combination.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

