All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Phase III SYMPATICO study on ibrutinib plus venetoclax in MCL
Ibrutinib is an oral inhibitor of Bruton tyrosine kinase (BTK) that received accelerated approval by the U.S. Food and Drug Administration (FDA) as a single-agent treatment for patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.1
The accelerated approval was based on complete response (CR) rates (19–21%) and overall response rates (68–72%) achieved in this setting. In addition, results from the phase III RAY trial (NCT01646021) showed that patients with MCL treated with ibrutinib had a better progression-free survival (PFS) compared with patients treated with temsirolimus.
To improve its efficacy, ibrutinib has been tested in combination with venetoclax, a BCL-2 inhibitor that has shown efficacy in MCL (CR rates, 21%) and is already approved in the US for patients with chronic lymphocytic leukemia (CLL) and for patients with previously untreated acute myeloid leukemia (AML).2 This combination showed:
- synergistic antitumor activity in preclinical models3
- good response rates in the phase II AIM study (NCT02471391) in patients with MCL, with a CR rate of 50% in patients with TP53 mutations (a population considered chemo-insensitive) and a median PFS of 29 months4 for the overall population
- high rates of CR in high-risk and older patients with previously untreated CLL (NCT02756897)
During the 8th annual meeting of the Society of Hematologic Oncology (SOHO 2020), Michael Wang reported on the ongoing SYMPATICO study (NCT03112174) evaluating ibrutinib plus venetoclax in patients with MCL.5
The phase III SYMPATICO study consists of three parts:5
- the safety run-in, evaluating the occurrence of tumor lysis syndrome (TLS) events and dose-limiting toxicities (DLTs) with the concurrent administration of ibrutinib and venetoclax
- the double-blind randomization, evaluating the efficacy of ibrutinib plus venetoclax versus ibrutinib plus placebo in patients with relapsed or refractory (R/R) MCL
- the open-label arm, designed to evaluate the efficacy and safety of ibrutinib plus venetoclax in patients with treatment-naïve MCL ≥ 65 years or < 65 years with a TP53 mutation
Safety run-in period5
Results from the safety run-in showed low rates of TLS events and DLTs:
- no clinical TLS events occurred
- three patients out of 21 (14%) had DLTs and all three were at high-risk for TLS
- Grade 4 neutropenia (n = 1)
- Grade 4 infection (n = 1)
- Grade 3 atrial fibrillation + Grade 3 hypotension (n = 1)
Efficacy results showed an overall response rate (ORR) of
- 76% in all patients, with a CR rate of 48%
- 73% in patients at high-risk for TLS, with a CR rate of 40%
- 83% in patients at low-risk for TLS, with a CR rate of 67%
Median PFS was not reached after a median follow-up of 10 months.
Double-blind randomization and open-label arm5
The randomized part of the study is fully enrolled, and patients are now being enrolled for the open-label arm.
The open-label arm is planned to be conducted in 75 patients, ≥ 65 years or 18─65 years with TP53 mutations, with previously untreated MCL. Key inclusion criteria also include: pathologically confirmed treatment-naïve MCL with either cyclin D1 overexpression in association with other relevant markers (e.g., CD19, CD20, PAX5, CD5) or evidence of t(11;14); at least one measurable disease site ≥ 2 cm; Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and formalin-fixed, paraffin-embedded tumor tissue submitted to the central laboratory. Patients who received prior treatment with a BTK or a BCL-2 inhibitor are ineligible.
In the frontline part, patients will receive oral ibrutinib + venetoclax for 24 months:
- ibrutinib, 560 mg once daily
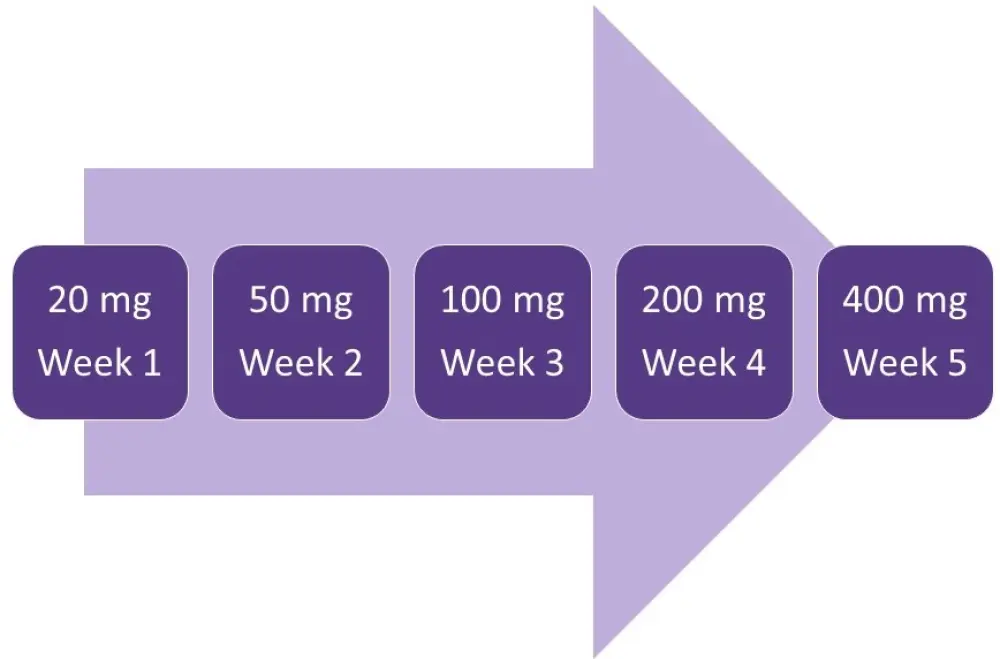
- venetoclax, once daily in a 5-week ramp-up to 400 mg (Figure 1)
Afterwards, venetoclax is discontinued and ibrutinib continued until progressive disease or unacceptable toxicity.
Figure 1. Venetoclax ramp-up5
The primary objective is to evaluate the CR rate. Secondary objectives include ORR, measurable residual disease-negative remission rate, PFS, overall survival, time to next treatment, safety, and pharmacokinetics.
Conclusion
Due to the promising results from the safety run-in part of the SYMPATICO trial, with low rates of TLS events and DLTs, this multicenter study is actively enrolling for the open-label arm at 46 sites across 17 countries.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

