All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Pirtobrutinib after a covalent BTK inhibitor in CLL/SLL
Do you know... Which of the following mutations has been associated with resistance to covalent BTK inhibitors but not pirtobrutinib?
Over the past decade, the introduction of highly effective and safe novel therapies such as B-cell lymphoma 2 (venetoclax) and covalent Bruton's tyrosine kinase (BTK) inhibitors (ibrutinib, zanubrutinib, and acalabrutinib) have significantly advanced the treatment landscape of chronic lymphocytic leukemia (CLL). However, while these BTK inhibitors have improved outcomes for patients with CLL/small lymphocytic leukemia (SLL), patients often develop resistance to these treatments and relapse. Emergent resistance against covalent BTK inhibitors is a growing issue in CLL, with 50–75% of patients acquiring a mutation of the target Cys481 residue at the adenosine triphosphate-binding pocket of BTK, rendering covalent BTK inhibitor's binding ineffective.
Pirtobrutinib is a selective, non-covalent BTK inhibitor which inhibits both wild-type and C481 mutated BTK, a common mutation involved in resistance to covalent BTK inhibitors, and is designed to overcome several limitations of covalent BTK inhibitors. It has recently been U.S. Food and Drug Administration (FDA) approved for relapsed/refractory (R/R) mantle cell lymphoma following two lines of systemic therapy including a BTK inhibitor.
The Lymphoma Hub previously reported promising outcomes of pirtobrutinib in relapsed B-cell malignancies and Waldenstrom’s macrobulinemia. Below, we summarize an article by Mato et al., published in New England Journal of Medicine, on the efficacy and safety of pirtobrutinib in R/R CLL/SLL following a BTK inhibitor.
Study design
BRUIN (NCT03740529) is a phase I/II trial of pirtobrutinib in patients with R/R CLL. The study was conducted across ten countries−Australia, France, Italy, Japan, Poland, South Korea, Sweden, Switzerland, United Kingdom, and the United States.
Patients received pirtobrutinib monotherapy in either phase I, at a dose range of 25–300 mg once daily in 28-day cycles, or phase II, at a dose of 200 mg once daily.
The primary endpoint was an overall response (OR), defined as a partial response or better according to the 2018 International Workshop on CLL response criteria. Secondary endpoints were OR including PR with lymphocytosis, progression-free survival, overall survival, and safety.
Results
Baseline characteristics
Of the 317 patients with CLL/SLL, 247 had previously received treatment with at least one BTK; including ibrutinib (n = 216), acalabrutinib (n = 44), zanubrutinib (n = 7), nemtabrutinib (n = 7), vecabrutinib (n = 3), spebrutinib (n = 3), and tirabrutinib (n = 1). Overall, 86 patients were treated in the phase I part, and 161 patients in the phase II portion. The median age was 69 years and median number of prior lines of therapy was three; baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
CAR, chimeric antigen receptor; cBTKi, covalent Bruton’s kinase inhibitor; CLL, chronic lymphocytic leukemia; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; SLL, small lymphocytic leukemia |
|
|
Characteristic, % (unless otherwise stated) |
Pirtobrutinib after cBTKi |
|---|---|
|
Median age (range), years |
69 (36–88) |
|
Sex |
|
|
Male |
68 |
|
Female |
32 |
|
Classification |
|
|
CLL |
99.6 |
|
SLL |
0.4 |
|
Rai stage |
|
|
0–II |
53.0 |
|
III or IV |
41.3 |
|
Bulky disease ≥5 cm |
31.6 |
|
ECOG Performance Status score |
|
|
0 |
53.8 |
|
1 |
39.3 |
|
2 |
6.9 |
|
Number of previous lines of systemic therapy |
|
|
Median (range) |
3 (1−11) |
|
1 |
7.7 |
|
2 |
22.3 |
|
3 |
23.1 |
|
≥4 |
47.0 |
|
Previous therapy |
|
|
BTK inhibitor† |
100 |
|
Anti-CD20 antibody |
87.9 |
|
Chemotherapy |
78.9 |
|
BCL2 inhibitor |
40.5 |
|
P13K inhibitor |
18.2 |
|
CAR T-cell therapy |
5.7 |
|
Allogeneic stem cell transplantation |
2.4 |
|
Median time from diagnosis to first dose of Pirtobrutinib (IQR) |
11 (8–15) |
|
Reason for discontinuation‡ |
|
|
Disease progression |
76.9 |
|
Toxic effects or other |
23.1 |
|
Mutation status |
|
|
BTK C481 |
|
|
mutated |
37.8 |
|
not mutated |
62.2 |
|
PLCG2 |
|
|
mutated |
8.1 |
|
not mutated |
91.9 |
|
High-risk molecular features§ |
|
|
17p deletion |
29.0 |
|
TP53 mutation |
39.2 |
|
17p deletion, TP53 mutation, or both |
46.6 |
|
Both 17p deletion and TP53 mutation |
28.2 |
|
Unmutated IGHV |
84.8 |
|
Complex karyotype‖ |
42.0 |
|
11q deletion |
25.0 |
Efficacy
The OR for patients who previously received a BTKi or BTKi plus BCL2 inhibitor are reported in Figure 1. The OR rate remained consistent across most subgroups by patient demographic characteristics, molecular features, or the extent of additional previous therapy. The majority of patients showed a decrease in the size of target lesions, regardless of the reason for discontinuation or previous BTKi/BCL2 inhibitor treatment.
Figure 1. Response rates*
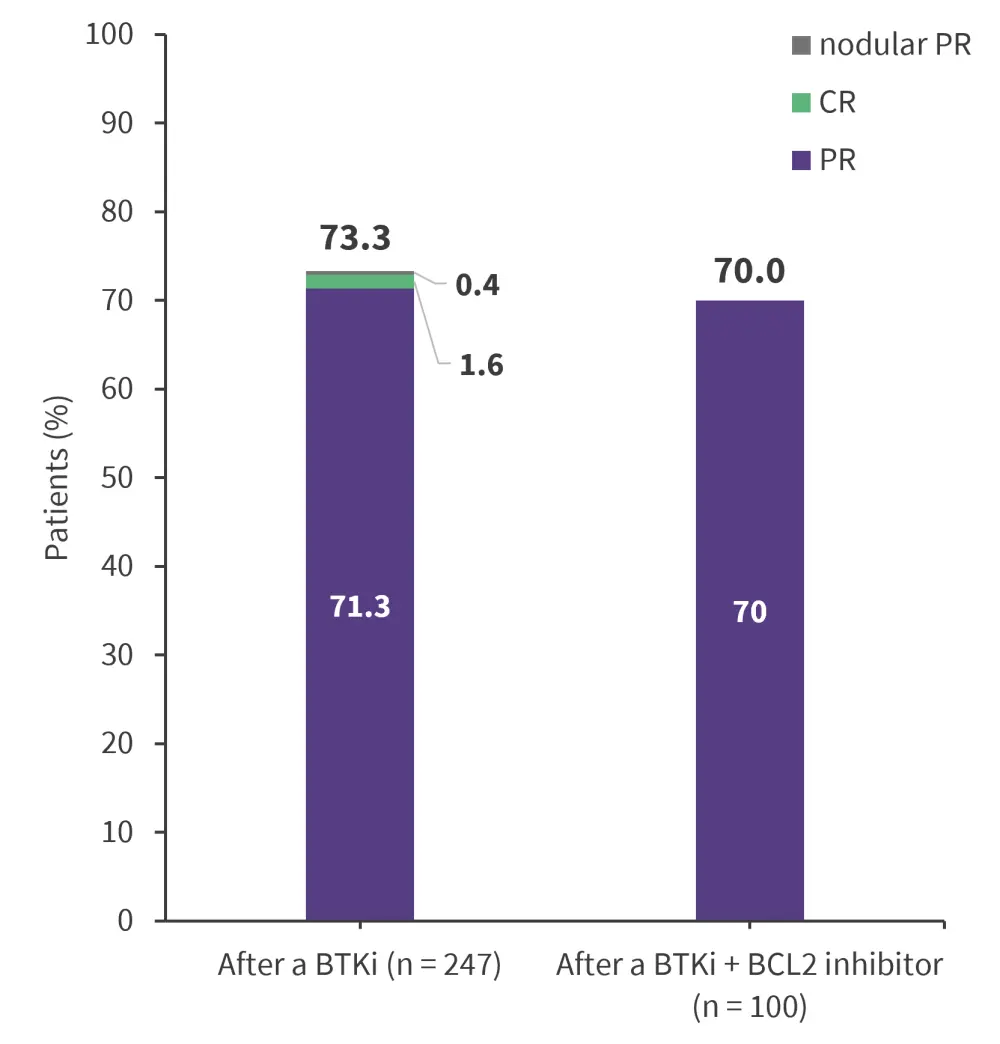
CR, complete response; PR, partial response.
*Data from Mato, et al.1
At a median follow-up of 19.4 months, the median progression-free survival across different therapies and subgroups are reported in Figure 2. At a median follow-up of 22.6 months, the 12-month and 18-month overall survival among patients who previously received a BTK inhibitor was 86.0% and 80.5%, respectively.
Figure 2. Median PFS*
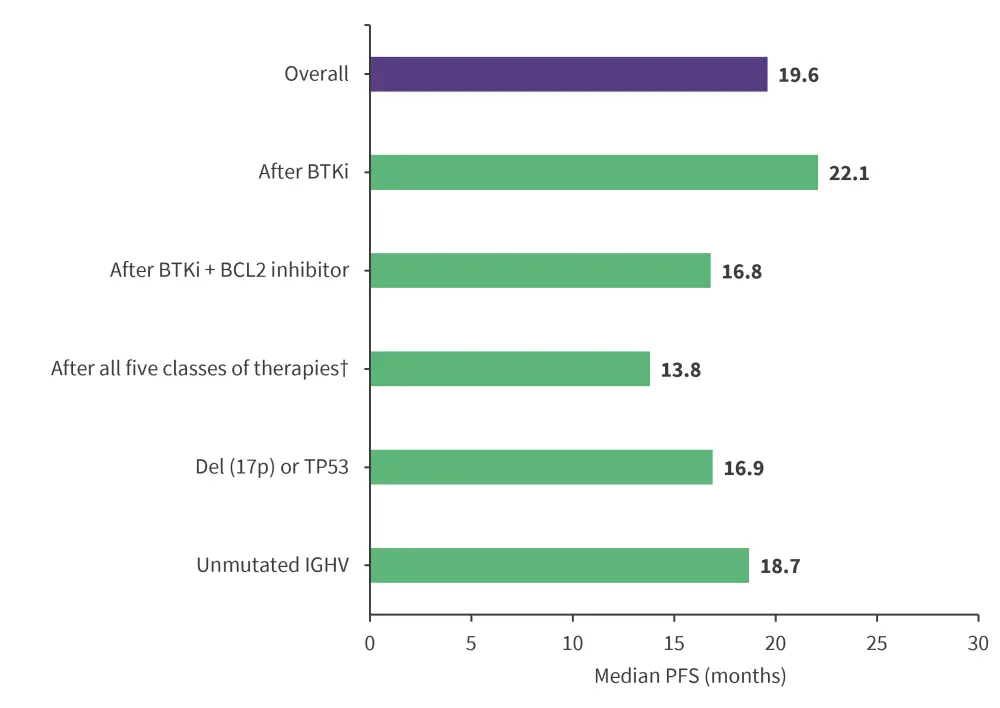
BCL2, B-cell lymphoma-2; BTKi, Bruton’s tyrosine kinase inhibitor; IGHV, immunoglobulin heavy chain; PFS, progression-free survival.
*Data from Mato, et al.1
†Therapies include BTKi, BCL2, and PI3K inhibitors as well as chemoimmunotherapy (chemotherapy and an anti-CD20 antibody).
Safety
Among the safety population (n = 317), the most common adverse events of special interest were infections, bleeding, and neutropenia (Table 2).
- No sudden cardiac deaths were reported.
- A total of 16 deaths were reported due to coronavirus disease-related pneumonia (n = 8), fungal pneumonia (n = 2), septic shock (n = 2), and other causes (n = 4).
- Treatment-related adverse events led to pirtobrutinib dose reductions in 35 patients and permanent discontinuations in 20 patients.
Table 2. AEs of special interest*†
|
AE, adverse event *Data from Mato, et al.1 |
||
|
AE, % |
Any grade |
Grade ≥3 |
|---|---|---|
|
Infections |
71.0 |
28.1 |
|
Bleeding |
42.6 |
2.2 |
|
Neutropenia |
32.5 |
26.8 |
|
Bruising‡ |
30.3 |
0 |
|
Hemorrhage |
21.1 |
2.2 |
|
Hypertension |
14.2 |
3.5 |
|
Atrial fibrillation or flutter§ |
3.8 |
1.3 |
Conclusion
Despite the lack of an active control group in this trial, pirtobrutinib proved effective and safe in patients with heavily pretreated CLL/SLL who experienced disease progression with a prior covalent BTK inhibitor; this demonstrates its potential as a therapeutic option, regardless of discontinuation due to disease progression, toxic effects, or other reasons.
There are several ongoing randomized phase III trials investigating pirtobrutinib in patients with CLL/SLL, including the BRUIN CLL-313 study (NCT05023980) evaluating pirtobrutinib versus bendamustine plus rituximab in untreated patients; the BRUIN-CLL-314 trial (NCT05254743) on pirtobrutinib versus ibrutinib; the BRUIN CLL-321 trial (NCT04666038) on pirtobrutinib versus either idelalisib plus rituximab or bendamustine plus rituximab; and the BRUIN CLL-322 study (NCT04965493) on pirtobrutinib plus venetoclax and rituximab versus venetoclax plus rituximab.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




