All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Polatuzumab vedotin in previously untreated DLBCL: An Asia subpopulation analysis from POLARIX trial
The phase III POLARIX study (NCT03274492) investigated polatuzumab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) versus standard of care (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHOP) as frontline treatment for patients with diffuse large B-cell lymphoma (DLBCL). Previously, the Lymphoma Hub has reported primary results from POLARIX and the Food and Drug Administration (FDA) approval of polatuzumab vedotin in combination with R-CHP in patients with previously untreated DLBCL. Below, we summarize results from the Asia subpopulation analysis of the POLARIX study investigating the safety and efficacy of polatuzumab vedotin combined with rituximab plus cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP).1
Study design
This was a double-blind, placebo-controlled, international phase III study. Previously untreated patients with DLBCL were included in the analysis from the Asia subpopulation and the China extension cohort. Patients included were
- aged 18–80 years;
- treatment naïve;
- diagnosed with CD20-positive DLBCL; and
- had an Eastern Cooperative Oncology Group Performance Status of 0 to 2, an International Prognostic Index (IPI) score between 2 and 5, and adequate organ function.
Patients were randomized to Pola-R-CHP (n = 141) and R-CHOP (n = 140). The study protocol, schedule of assessments, and treatment were the same across patients analyzed in this subpopulation and the global cohort; stratification factors included IPI score and bulky disease, defined as absent or present with a lesion >7.5 cm. The primary endpoint was progression-free survival (PFS) and the primary safety endpoint was the incidence of adverse events (AEs).
Results
Overall, 281 patients were included in the study (Asia subpopulation, n = 160; China extension cohort, n = 121). The patient’s baseline demographics and clinical characteristics were balanced across the treatment arms (Table 1).
Table 1. Baseline patient demographics and clinical characteristics (intention-to-treat population)*
|
ECOG, Eastern Cooperative Oncology Group; IPI score, integrated pulmonary index; Pola-R-CHP, polatuzumab vedotin combined with rituximab plus cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. *Data from Song, et al.1 |
||
|
Characteristic, % (unless otherwise stated) |
Pola-R-CHP (n = 141) |
R-CHOP (n = 140) |
|---|---|---|
|
Median age range, years |
19–79 |
23–78 |
|
Age |
|
|
|
<65 years |
58.9 |
52.1 |
|
≥65 years |
50.4 |
55.7 |
|
Country |
|
|
|
Mainland China |
53.2 |
53.6 |
|
Japan |
30.5 |
30.0 |
|
Republic of Korea |
10.6 |
11.4 |
|
Taiwan |
5.7 |
5.0 |
|
Ann Arbor stage |
|
|
|
I–II |
13.5 |
15.0 |
|
III–IV |
86.5 |
85.0 |
|
Extranodal sites |
|
|
|
0–1 |
82.3 |
86.4 |
|
2 |
17.7 |
13.6 |
|
Bulky disease† |
|
|
|
<7.5 cm |
68.8 |
66.4 |
|
≥7.5 cm |
31.2 |
33.6 |
|
ECOG Performance status |
|
|
|
0–1 |
82.3 |
86.4 |
|
2 |
17.7 |
13.6 |
|
LDH level |
|
|
|
Normal |
31.9 |
29.3 |
|
Elevated |
68.1 |
70.7 |
|
Median time from initial diagnosis to treatment initiation (IQR), days |
17 (10–29) |
15 (10–26) |
|
IPI score, %† |
|
|
|
2 |
38.3 |
37.9 |
|
3–5 |
61.7 |
62.1 |
|
Cell of origin‡ |
|
|
|
GCB |
30 |
40 |
|
ABC |
55 |
46 |
|
Unclassified |
15 |
14 |
|
DEL‡ |
|
|
|
DEL |
31.8 |
36.3 |
|
Non-DEL |
68.2 |
63.7 |
|
Double/triple hit lymphoma‡ |
|
|
|
Yes |
2.9 |
5.1 |
|
No |
97.1 |
94.9 |
|
Previous history of hepatitis B infection§ |
32.1‖ |
42.4 |
Efficacy
- The study met its primary endpoint of PFS, consistent with the global study population (hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.40–1.03). Primary and key secondary efficacy outcomes are shown in Figure 1.
- In the subpopulation analysis, 74.2% and 66.5% remained progression free at 2 years in the pola-R-CHP and R-CHOP arm, respectively.
- Fewer patients receiving Pola-R-CHP experienced disease relapse and received ≥1 subsequent anti-lymphoma therapy compared with patients receiving R-CHOP (22% vs 32.9%).
Figure 1. Primary and key secondary efficacy outcomes (intention-to-treat population)*
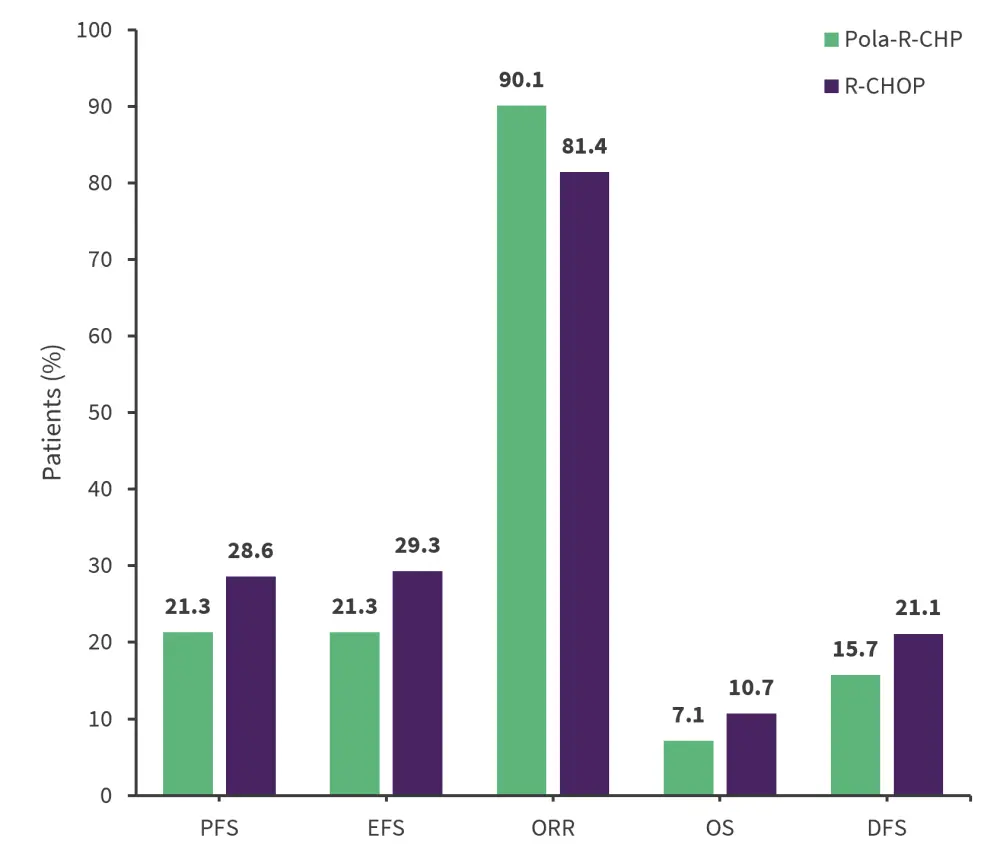
DFS, disease free survival; EFS, even free survival; ORR, objective response rate; OS, overall survival; Pola-R-CHP, polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
*Adapted from Song, et al.1
Safety
- The most common treatment-emergent Grade 3/4 AEs were neutropenia, neutrophil count decreased, and white blood cell count decreased (Figure 2)
- Grade 5 AEs were reported by two patients in Pola-R-CHP arm (suspected COVID-19 and unexplained death) and one patient in the R-CHOP arm (unexplained death)
- The proportion of patients requiring treatment discontinuation was 5.0% in the Pola-R-CHP arm and 7.2% in the R-CHOP arm
Figure 2. Treatment-emergent Grade 3/4 AEs (safety-evaluable population)*
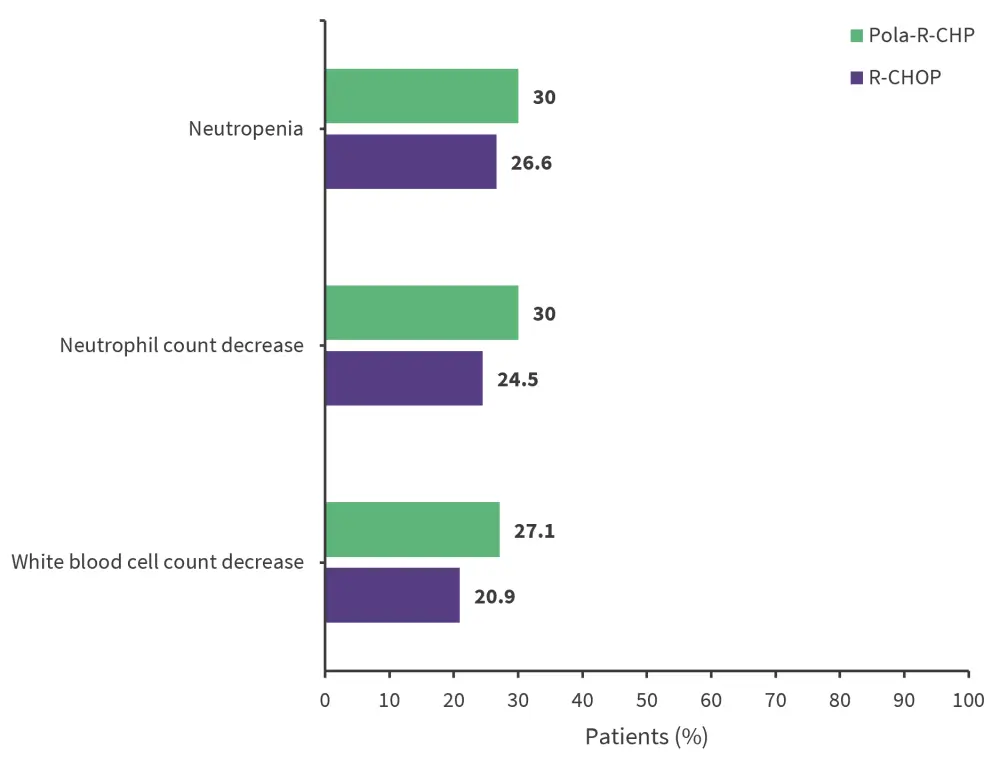
AE, adverse event; Pola-R-CHP, polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
*Adapted from Song, et al.1
Conclusion
These findings demonstrate the consistent efficacy and safety of Pola-R-CHP vs R-CHOP in the Asia and global populations of previously untreated patients with DLBCL enrolled in the POLARIX study. Of note, the subpopulation analysis is limited by a considerably smaller cohort and variability in follow-up compared with the entire study population.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



