All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Survival analysis of axi-cel versus standard of care in patients with R/R LBCL: ZUMA-7 trial
Historically, the standard of care (SOC) second-line treatment in patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) who respond to salvage chemoimmunotherapy has been high-dose chemotherapy and autologous stem cell transplantation (ASCT). However, only 50% of patients are eligible for this regimen, with approximately 20% being cured. Additionally, poor outcomes are seen in patients ineligible for high-dose chemotherapy and ASCT, with a median overall survival (OS) of 4.4 months.1
Axicabtagene ciloleucel (axi-cel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, was previously approved as a third or later-line therapy for R/R LBCL based on the ZUMA-1 trial (NCT02348216) results. However, the phase III ZUMA-7 trial (NCT03391466) showed a higher event-free survival (EFS) and objective response rate with axi-cel as second-line treatment in patients with early R/R LBCL compared with SOC; these results led to the approval of axi-cel by the U.S. Food and Drug Administration (FDA) in April 2022.1
We are pleased to summarize a recent survival analysis of ZUMA-7 by Westin et al.,1 published in New England Journal of Medicine.
Study design
This phase III randomized trial included adult patients aged ≥18 years who had histologically confirmed LBCL, were refractory to first-line therapy, or had relapsed within 12 months of first-line chemoimmunotherapy. Eligible patients were randomized 1:1 to receive axi-cel or SOC. The SOC schedule involved two or three cycles of investigator-selected chemoimmunotherapy followed by high-dose chemotherapy and ASCT in patients who had a complete or partial response.
The primary endpoint was EFS, defined as the time from randomization to disease progression (according to Lugano classification), start of new therapy, or death from any cause.
Key secondary endpoints were objective response and OS. Other secondary endpoints included investigator-assessed progression-free survival (PFS), defined as time from treatment onset to disease progression (according to Lugano classification) or death from any cause, and investigator-assessed EFS.
Results
Baseline characteristics1
A total of 359 patients were included, with 180 and 179 patients in axi-cel and SOC arm, respectively. The baseline characteristics were similar between the two treatment arms, including a larger proportion of non-Hispanic White patients compared with other racial or ethnic groups.
Most patients had high-risk features, including
- 74% with primary refractory disease;
- 79% with stage III or IV disease;
- 30% aged ≥65 years; and
- 19% with high-grade B-cell lymphomas, including double-hit lymphomas according to investigators assessment.
Efficacy1
At a median follow-up of 47.2 months, axi-cel significantly improved OS (p = 0.03) compared with SOC across all patient subgroups, resulting in a 27.4% reduction in the risk of death. The median OS was not reached in the axi-cel arm compared with 31.1 months in the SOC arm.
Axi-cel showed an improved investigator-assessed PFS compared with SOC, with a median PFS of 14.7 vs 3.7 months, respectively. Similarly, investigator-assessed EFS was longer for axi-cel versus SOC at 10.8 vs 2.3 months, respectively. The estimated 4-year OS, EFS, and PFS rates are summarized in Figure 1.
Figure 1. Estimated 4-year survival outcomes*
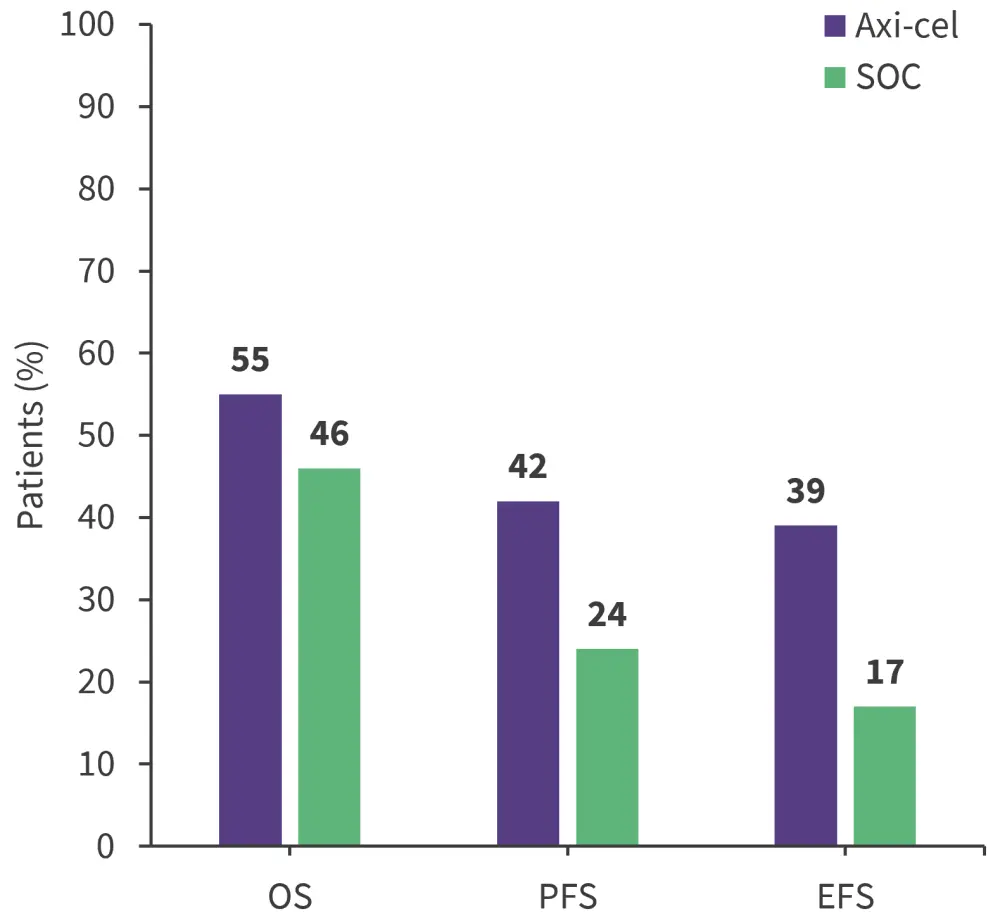
axi-cel, axicabtagene ciloleucel; EFS, event-free survival; OS, overall survival; PFS, progression-free survival; SOC, standard of care
*Data from Westin, et al.1
Safety1
In the safety analysis (axi-cel, n = 170; SOC, n = 168), all patients experienced at least one adverse event (AE).
- A total of 74 and 91 deaths were reported in the axi-cel and SOC arm, respectively; the most common cause of death was progressive disease in both arms.
- Any grade infections occurred in 45% and 32% of patients in the axi-cel and SOC arm; and Grade ≥3 infections occurred in 17% and 12%, respectively.
- Grade ≥3 serious AEs occurred in 46% and 40% in the axi-cel vs SOC arm, respectively.
- No new treatment-related serious/fatal AEs, cytokine release syndrome, or neurologic events were reported since the prior analysis.
- New or secondary cancers were reported in eight and three patients in the axi-cel and SOC arm.
- B-cell aplasia was reported in 62% and 23% at 3 and 24 months, respectively, in patients in the axi-cel arm after infusion.
- Grade ≥3 AEs occurred in 91% and 83% of patients in axi-cel vs SOC arm; neutropenia was the most common AE reported across both arms (Figure 2).
Figure 2. Most common Grade 3≥ AEs reported in at least 20%*
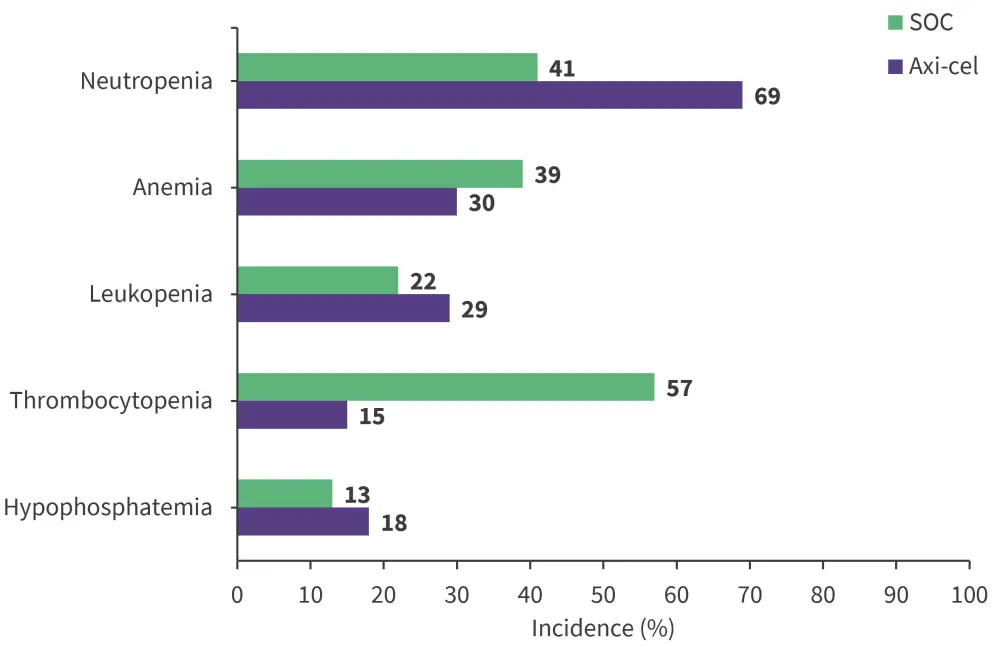
SOC, standard of care
*Data from Westin, et al.1
Exploratory analysis
Exploratory analyses showed that, except for greater proportion of juvenile or stem memory T-cell phenotype cells and lower proportion of differentiated T cells, the improved OS benefit in the axi-cel arm was independent of CAR T-cell characteristics. Peak CAR T-cell levels were not associated with OS.
Conclusion
This survival analysis of the ZUMA-7 trial demonstrates the significant OS benefit of axi-cel compared with SOC as a second-line curative treatment for patients with early R/R LBCL, including older adults. The safety profile of axi-cel was consistent with the previous analysis. A limitation of this study is the lack of diverse racial and ethnic groups; therefore, future trials should aim to increase diversity, further improve patient outcomes, and investigate treatment-related toxicities. Real-world data on axi-cel are needed to further establish these findings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content





