All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
SYMPATICO study safety run-in: simultaneous ibrutinib plus venetoclax for R/R MCL
Mantle cell lymphoma (MCL) is an aggressive B-cell non-Hodgkin lymphoma lacking therapeutic options that prolong progression free and overall survival. In recent years, targeted agents such as ibrutinib, a once-daily Bruton’s tyrosine kinase inhibitor, have been transformative for patients with relapsed/refractory (R/R) MCL and have partly replaced conventional chemotherapy. Venetoclax, an oral B-cell lymphoma 2 inhibitor, is highly effective at initiating apoptosis, but is also associated with an increased risk of tumor lysis syndrome (TLS). To mitigate this possibility, a lead-in with a tumor-debulking agent, such as an anti-CD20 antibody or single-agent ibrutinib, is administered. The combination of ibrutinib plus venetoclax has demonstrated encouraging clinical activity in early phase studies for MCL.
The safety and efficacy of concurrently administered ibrutinib plus venetoclax is being evaluated by Wang, et al. in the ongoing SYMPATICO (NCT03112174) study for patients with R/R MCL.1 We have previously summarized the SYMPATICO study, and herein we provide the updated safety run-in results.
Study design
SYMPATICO is a phase III, multinational study comprising an open-label safety run-in cohort and a double-blind, randomized period, both conducted in patients with R/R MCL.
Eligibility criteria (safety run-in cohort)
- Aged ≥18 years
- Pathologically confirmed MCL
- One or more sites of disease measuring ≥2.0 cm
- One to five prior therapies for MCL (including at least one prior rituximab/anti-CD20-containing regimen)
- Eastern Cooperative Oncology Group (ECOG) Performance Status of 0–2
Exclusion criteria
- Prior treatment with Bruton’s tyrosine kinase or B-cell lymphoma 2 inhibitors
Primary endpoint
- The primary endpoint of the safety run-in cohort was the occurrence of TLS and dose-limiting toxicity (DLT) events.
Secondary endpoints
- overall response rate
- progression free survival (PFS)
- duration of response
- safety
Patients were treated concurrently with oral ibrutinib 560 mg once daily and venetoclax starting from 20 mg once daily and ramped up over 5 weeks to a target dose of 400 mg. Ibrutinib plus venetoclax was administered concurrently for 2 years followed by once-daily, single-agent ibrutinib until disease progression, unacceptable toxicity, or withdrawal of consent.
TLS-risk categories were based on tumor burden. Patients with high tumor burden (≥1 lesion, >10 cm or ≥1 lesion, >5 cm with circulating lymphocytes >25,000 cells/mm3) and/or creatinine clearance <60 mL/min at baseline were considered at increased risk for TLS and received TLS prophylaxis and monitoring.
DLT was defined as any Grade ≥3 non-TLS adverse event (AE) at least possibly related to ibrutinib and/or venetoclax occurring during the 5 week ramp-up period, which ended on Day 7 of venetoclax 400 mg. Minimal residual disease (MRD) was assessed in bone marrow and peripheral blood by flow cytometry.
Results
The safety run-in cohort enrolled 21 patients (Table 1). All patients had confirmed progressive disease and at least one lesion measuring >2 cm at baseline; 52% of the patients had baseline detectable MRD. For patients with available TP53 mutation data, 38% (5/13) had mutated TP53. Median follow up was 31 months.
Table 1. Baseline patient and disease characteristics*
|
TLS, tumor lysis syndrome. |
|||
|
Characteristic |
Patients at low risk |
Patients at increased |
All patients |
|---|---|---|---|
|
Median age (range), years |
62 (54–67) |
70 (53–84) |
68 (53–84) |
|
Age category, % |
|||
|
<60 |
33 |
7 |
14 |
|
60–69 |
67 |
33 |
43 |
|
≥70 |
0 |
60 |
62 |
|
Male, % |
67 |
60 |
62 |
|
Median longest diameter |
3 (2–5) |
8 (2–15) |
4 (2–15) |
|
Median circulating |
1.7 (0.1–3.8) |
1.1 (0.4–83.9) |
1.2 (0.1–83.9) |
|
Median creatine clearance |
108 (72–140) |
57 (36–94) |
70 (36–140) |
|
Median number of prior |
2 (1–2) |
2 (1–4) |
2 (1–4) |
Safety analysis
The median treatment duration was 20.1 months, and there were no clinical TLS events. However, one 74-year-old female at increased risk for TLS had laboratory TLS on Day 2, with the following laboratory values:
- potassium (maximum of 4.6 mmol/L) and calcium (minimum of 1.89 mmol/L) within the normal range
- elevated phosphorus (maximum of 2.84 mmol/L)
- elevated uric acid (maximum of 667 µmol/L)
- elevated creatinine (maximum of 137 µmol/L)
The patient had medical history of Grade 1 hypertension, Grade 2 atrial fibrillation, cardiac failure, chronic renal insufficiency, dyspnea, and lower extremity edema. Her baseline bone marrow lymphoma involvement was 10%, and her baseline white blood cell count was 111.9 × 109/L. The TLS event lasted 5 days, during which the patient remained on ibrutinib (560 mg) while venetoclax was held. On Day 7, the patient resumed venetoclax (20 mg); her white blood cell count gradually decreased to 5.8 × 109/L on Day 22, and ramp-up to full dose was achieved, with partial response.
DLTs occurred in three patients (14%): Grade 4 neutropenia lasting >7 days, Grade 4 infection, and Grade 3 atrial fibrillation plus Grade 3 hypotension. Most AEs were Grade 1/2. The most common Grade 3/4 AEs are illustrated in Figure 1 below. Grade ≥3 atrial fibrillation and Grade ≥3 hemorrhage occurred in one patient each. Five patients (24%) discontinued both study drugs due to AEs, and four patients (19%) discontinued treatment due to progressive disease.
Figure 1. Grade 3/4 adverse events occurring in >5% of patients*
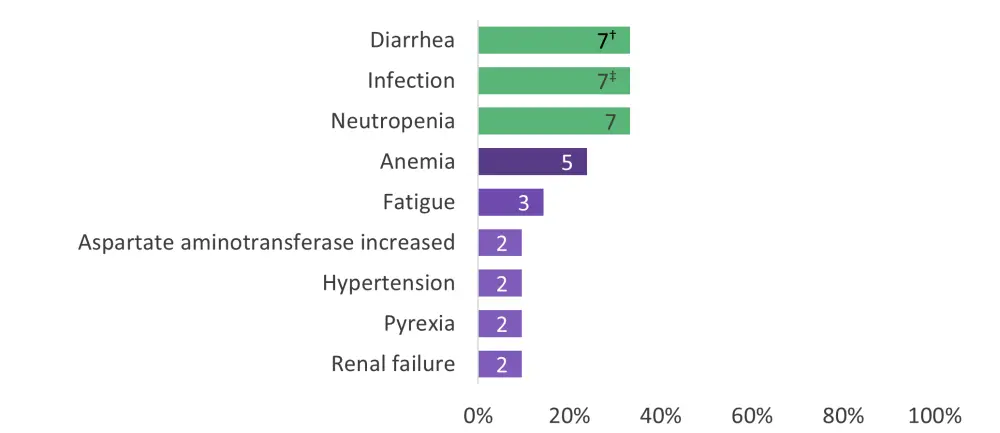
*Adapted from Wang, et al.1
†Patient numbers are shown within the bars.
‡Bronchitis (1), candida infection (1), cellulitis (1), fungal abscess central nervous system (1, recovered), infection (not specified, 1), pneumonia (2), sepsis (1), staphylococcal bacteremia (1), upper respiratory tract infection (1), and urinary tract infection (1).
Efficacy analysis
- Overall response rate was 81% (95% confidence interval [CI], 58–95%), and rates were similar regardless of TLS risk (see Figure 2).
- In total, 13 patients (62%) achieved a complete response (95% CI, 38–82%), and all 11 patients with detectable MRD at baseline achieved undetectable MRD.
- Median duration of response was 32.3 months (95% CI, 26.5–not estimable [NE]).
- Median PFS was 35.0 months (95% CI, 13.7–NE).
- The 30-month PFS estimate was 60% (95% CI: 31–80%).
- Median OS was also 35.0 months (95% CI, 20.7–NE).
Figure 2. Overall response by TLS-risk group*
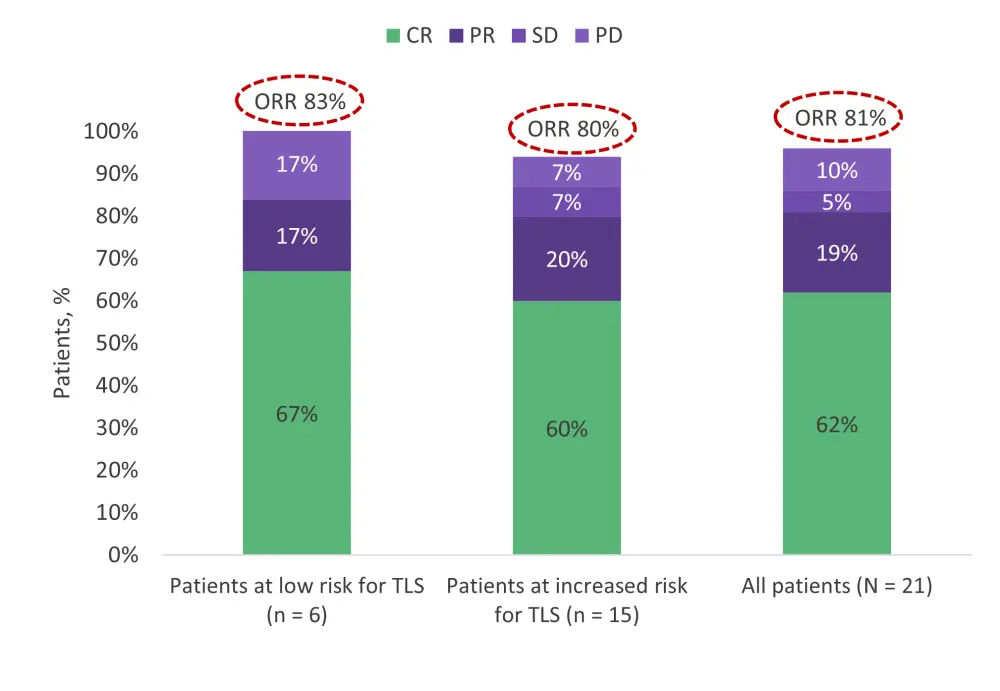
CR, complete response; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; TLS, tumor lysis syndrome.
*Adapted from Wang, et al.1
Conclusion
In conclusion, results from the safety run-in data demonstrate that the randomized portion should proceed with concurrent administration of ibrutinib and venetoclax, with no ibrutinib lead-in for patients with R/R MCL. The once-daily, all-oral, chemotherapy-free combination of ibrutinib plus venetoclax showed promising efficacy and tolerability with no new added toxicities. There were no cases of clinical TLS and the DLT incidence did not exceed the prespecified threshold to demand an ibrutinib lead-in.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

