All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Symposium | Sequencing therapies and practical management of adverse events in MCL
Featured:
Do you know... Which of the following is true of non-covalent BTK inhibitors, when compared with covalent BTK inhibitors?
During the 18th International Conference on Malignant Lymphoma (ICML), June 17–21, 2025, Lugano, CH, the Lymphoma Hub held a symposium on June 17, 2025, titled, Customizing therapy for mantle cell lymphoma (MCL). Here, we share a case-based presentation and panel discussion led by Wojciech Jurczak, Maria Skłodowska-Curie National Research Institute of Oncology, Kraków, PL, on sequencing therapies and practical management of adverse events in MCL.
Symposium | Sequencing therapies and practical management of adverse events in MCL
Symposium | Sequencing therapies and practical management of adverse events in MCL
Patient case 1
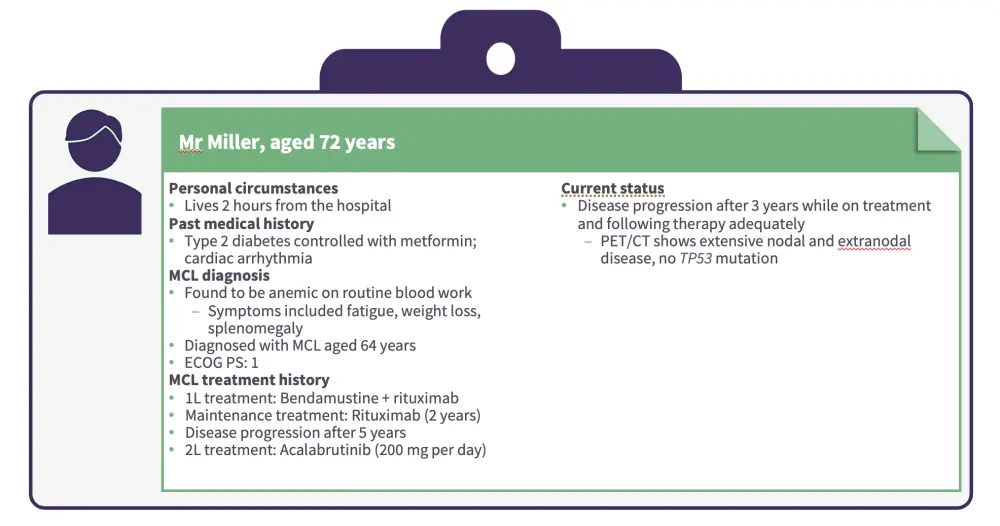
Jurczak first presented a patient case of a male aged 72 years with MCL who progressed while on treatment with second-line acalabrutinib (Figures 1 and 2).
Figure 1. Patient case 1
In the phase I/II BRUIN trial (NCT03740529), pirtobrutinib monotherapy demonstrated meaningful response rates (overall response rate [ORR], 49.3%; complete response [CR] rate, 15.8%) in patients with covalent Bruton’s tyrosine kinase inhibitor (cBTKi) relapsed/refractory (R/R) MCL.1
Median duration of response (DoR), median progression-free survival (PFS), and median overall survival (OS) were 21.6 months, 5.6 months, and 23.5 months, respectively.1
Responses were durable across high-risk subgroups, including Ki-67 index ≥30% (median PFS, 5.3 months) and mutated TP53 (median PFS, 3.7 months).1
Pirtobrutinib was well-tolerated with low rates of adverse events typically associated with cBTKis.1
Pirtobrutinib is a highly selective and highly efficient BTKi for patients with MCL, with more potent BTK inhibition and less off-target activity, potentially reducing toxicity compared with covalent BTKis.2,3
Figure 2. Patient case 1 continued
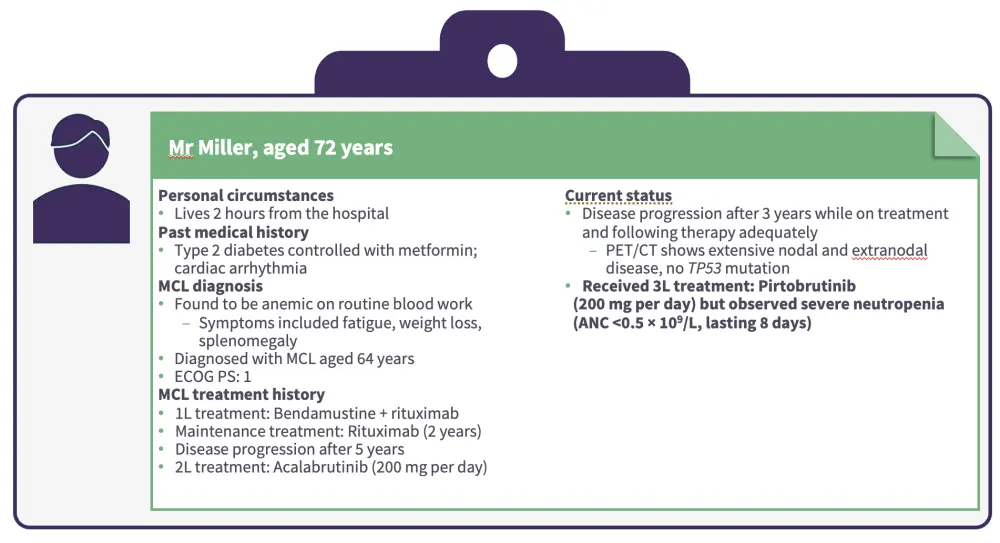
The recommended dose modification for hematologic adverse events at the first occurrence is to interrupt pirtobrutinib dosing until recovery to Grade 1 or baseline and then to restart pirtobrutinib at the original dose.4
Granulocyte colony-stimulating factor (G-CSF) support may also be useful for patients with neutropenia.
The panel then discussed factors influencing treatment selection of pirtobrutinib vs chimeric antigen receptor (CAR) T-cell therapy, monitoring and managing cardiac risks in older patients, and balancing efficacy vs tolerability in older patients with comorbidities. Key discussion topics included:
The impact of age, distance from the hospital, and other healthcare issues on the selection of pirtobrutinib vs CAR T-cell therapy.
The need to consider combination therapy or CAR T-cell therapy in patients with higher-risk features, such as a high Ki-67 proliferation index or mutated TP53.
The importance of considering next steps in the case of no response or loss of response after single-agent pirtobrutinib, such as switching to CAR T-cell therapy.
Patient case 2
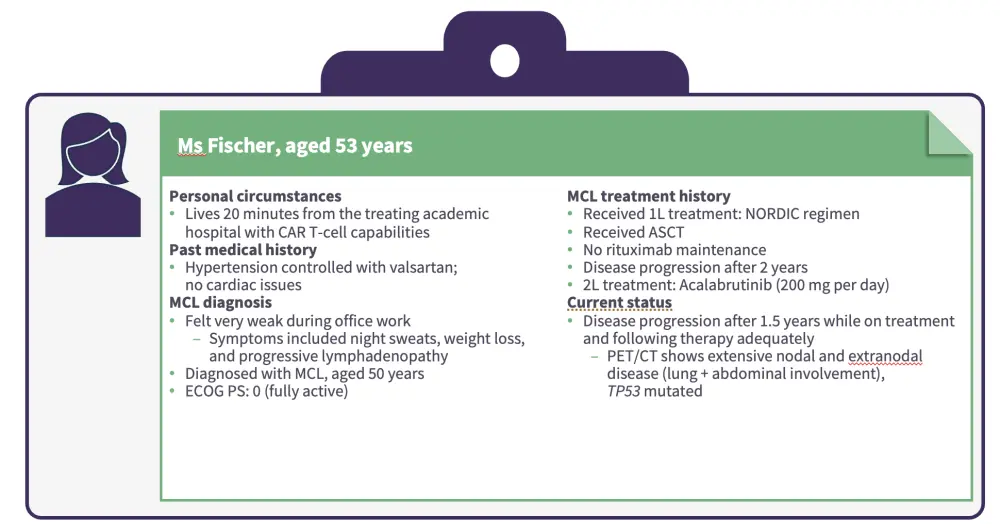
Jurczak then presented a patient case of a female aged 53 years with MCL who progressed while on treatment with second-line acalabrutinib (Figures 3 and 4).
Figure 3. Patient case 2
In the phase II ZUMA-2 trial (NCT02601313), brexucabtagene autoleucel (brexu-cel) demonstrated substantial and durable clinical benefit in patients with R/R MCL.5
After a median follow-up of 17.5 months, median DoR, PFS, and OS were not reached.5
The 15-month DOR, PFS, and OS rates were 59%, 59%, and 76%, respectively.5
Figure 4. Patient case 2 continued
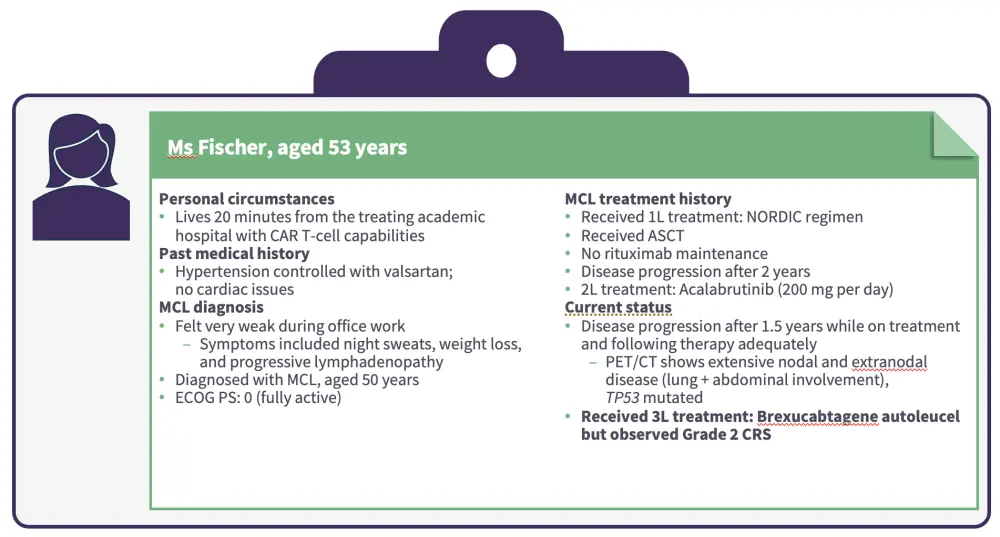
Based on results from several real-world studies, any-grade cytokine release syndrome (CRS) occurs in 87.9–95% of patients treated with brexu-cel (Grade 3 CRS, 8–21%).6
Any-grade immune effector cell-associated neurotoxicity syndrome (ICANS) occurs in 48–61% (Grade 3 ICANS, 15.4–32%).6
Non-relapse mortality rates range from 7.3% to 14.5%.6
The panel then discussed factors that determine eligibility for and timing of administration of CAR T-cell therapies, monitoring for CRS and ICANS, and how to incorporate bridging therapy for patients with MCL awaiting CAR T-cell therapy. Key discussion points included:
Younger age and access to CAR T-cell therapies are important factors to consider when selecting a therapy for patients who have failed two prior lines of therapy, including a BTKi.
Pirtobrutinib is an option as a bridging therapy to CAR T-cell therapy for patients with R/R MCL after a cBTKi.
Radiation therapy and chemotherapy may be useful as bridging therapies.
Jurczak concluded by highlighting the need to consider prior therapies and response to previous therapies, disease characteristics including molecular and genetic features, and patient-related factors when choosing a therapy in the R/R MCL setting.
This educational resource is independently supported by Eli Lilly and Company. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Wojciech Jurczak
Wojciech Jurczak
.webp&w=3840&q=75)
.webp&w=3840&q=75)
.webp&w=3840&q=75)