All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Tocilizumab for the management of cytokine release syndrome: Real-world data
Tocilizumab, an interleukin-6 inhibitor, is indicated for the treatment of chimeric antigen receptor (CAR) T-cell-associated cytokine release syndrome (CRS).1 During the European Society for Blood and Marrow Transplantation-European Hematology Association (EBMT-EHA) 6th European CAR T-cell meeting, Delaney1 presented real-world data on the use of tocilizumab for the management of CAR T-cell-associated CRS; we have summarized the presentation below.
Methods
- This analysis included 10 patients who received CAR T-cell therapy at the Sheffield Teaching Hospitals NHS Foundation Trust.
- Six patients with large B-cell lymphoma received axicabtagene ciloleucel, and four patients with mantel cell lymphoma received brexucabragene autoleucel.
- Tocilizumab treatment for CRS is shown in Figure 1.
- Time to response was defined as the time for a patient to drop ≥1 CRS grade.
- Grade 1 CRS was defined as the time for the patient’s temperature to return to normal.
- Grade 2 CRS was defined as the time for the patient’s blood pressure to return to normal without needing fluid support or for their oxygen saturation to return to >94% without supplemental oxygen.
- Patients with Grade ≥3 CRS were not included in the response time analysis.
Figure 1. Sheffield Teaching Hospitals reference guide to CRS management*
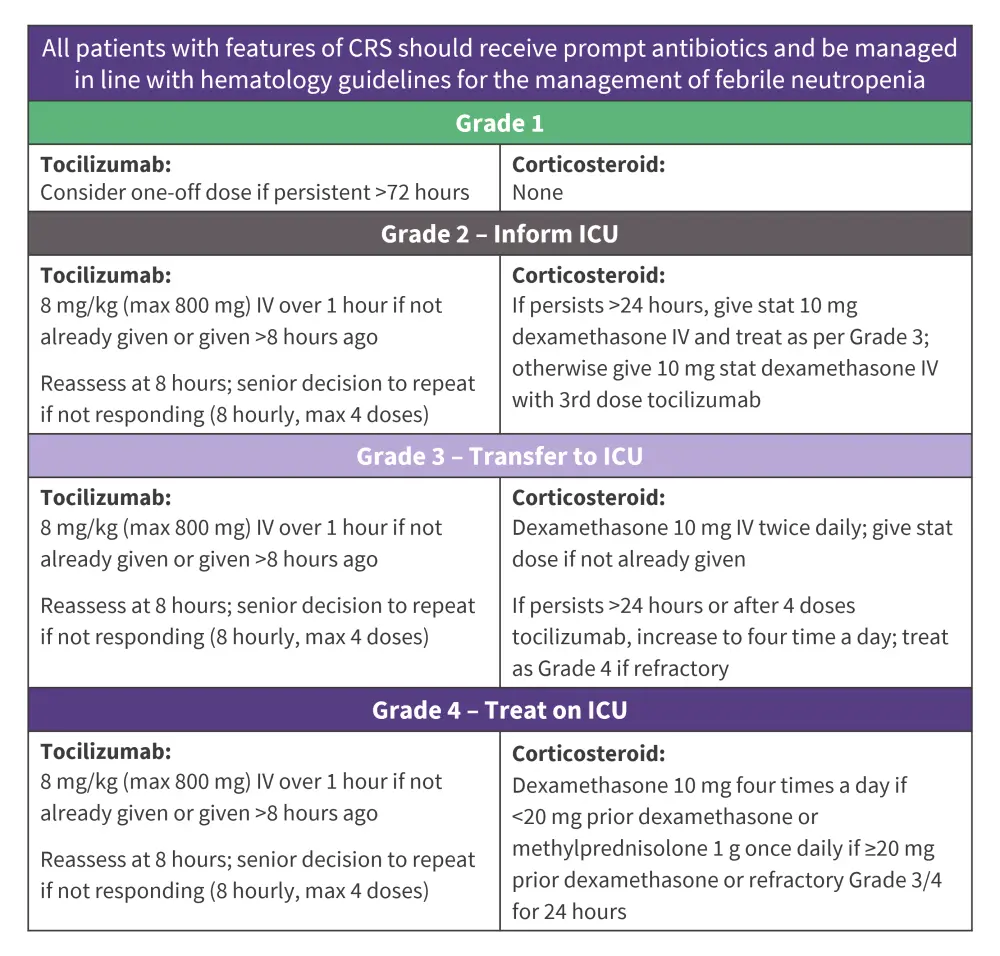
CRS, cytokine release syndrome; ICU, intensive care unit; IV, intravenously.
Adapted from Delaney.1
Key findings
- In total, nine patients developed CRS:
- Median day of CRS onset was Day 1 (range, 1–8);
- Average CRS duration was 5.5 days (range, 1–8.5); and
- Median day of maximum CRS was Day 4 (range, 1–8).
- Three patients developed Grade 3 CRS requiring vasopressors, of which two were aged >70 years, and two developed Grade 3 immune effector cell-associated neurotoxicity syndrome.
- Two patients received one dose, two received two doses, three received three doses, and two received four doses of tocilizumab.
- Median administration of the first tocilizumab dose was on Day 2 (range, 1–9).
- Average response times were similar between the first and second tocilizumab doses but increased with further doses (Figure 2).
- Response times were faster for Grade 2 vs Grade 1 after the first and second doses; however, additional resuscitative measures were also given (Figure 2).
- Of the two patients who received their first tocilizumab dose for Grade 1 CRS at 24 hours, response time was relatively long at 11 and 15 hours, respectively, and this did not prevent higher grade CRS or needing further tocilizumab doses.
Figure 2. Average tocilizumab response time*
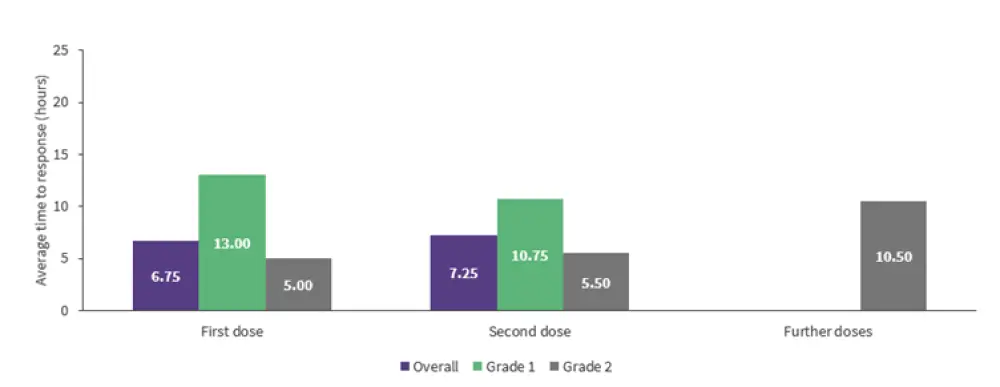
*Data from Delaney.1
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



