All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Using ctDNA analysis in risk profiling of frontline DLBCL: Results from the POLARIX study
As previously reported on the Lymphoma Hub, the primary analysis of the phase III POLARIX (NCT03274492) study showed a higher progression free survival (PFS) benefit for polatuzumab vedotin with R-CHP (Pola-R-CHP) arm vs R-CHOP (2-year PFS difference, 6.5%) alone in patients with previously untreated diffuse large B-cell lymphoma (DLBCL) and an International Prognostic Index (IPI) of 2–5; safety profiles were comparable between both arms.1
Prior studies have shown the prognostic relevance of circulating tumor DNA (ctDNA) in patients with DLBCL, with one study demonstrating improved outcomes at a 2.0 ctDNA log-fold change (LFC) after one induction cycle.1
In this article, we summarize a prespecified exploratory analysis examining the prognostic value of ctDNA in the POLARIX study, as presented by Herrera at the 64th American Society of Hematology (ASH) Annual Meeting and Exhibition in 2022.1
Study design
The POLARIX study included patients with previously untreated intermediate- to high-risk DLBCL. Patients were randomized to receive pola-R-CHP or R-CHOP, with plasma samples collected at various timepoints for ctDNA assessment (Figure 1).
Figure 1. Inclusion criteria, treatment schema, and ctDNA collection schedule in POLARIX study*
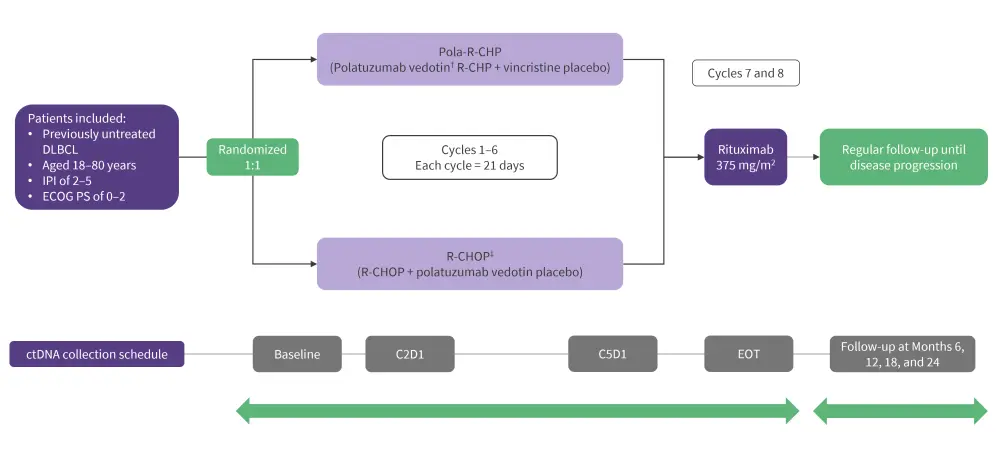
C2D1, Cycle 2 Day 1; C5D1, Cycle 5 Day 1; DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EOT, end of treatment; IPI, International Prognostic Index; R, randomized; R-CHP, rituximab, cyclophosphamide, doxorubicin, and prednisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
*Data from Herrera, et al.1
†Intravenous polatuzumab vedotin: 1.8 mg/kg body weight.
‡RCHOP: Rituximab, 375 mg/m2 intravenous; cyclophosphamide, 750 mg/m2 intravenous; doxorubicin, 50 mg/m2 intravenous; vincristine, 1.4 mg/m2; prednisone, 100 mg oral.
ctDNA assessment1
- Plasma ctDNA levels were measured at baseline and Day 1 of Cycle 2 (C2D1) using the AVENIO NHL CAPP-Seq assay;
- ctDNA levels were expressed as mean mutant molecules per mL (MMPM);
- Plasma-depleted whole blood at baseline was used as a source of germline DNA to filter non-tumor-specific variants;
- LFC in ctDNA were defined as the log10 ratio between baseline and C2D1 MMPM; and
- ctDNA clearance was determined using a detection cutoff of p = 0.005.
Results
Baseline characteristics were similar between the two treatment arms (pola-R-CHP and R-CHOP) in the intention to treat (ITT) population.
Updated survival analysis1
At the longer median follow-up of 37.9 months,
- Pola-R-CHP demonstrated a sustained PFS benefit when compared to R-CHOP alone (3-year PFS difference, 7.7%);
- the OS rates remained similar between the two arms; and
- no new safety signals were observed.
ctDNA analysis
Of the 879 patients in the ITT population, 621 were included in the ctDNA biomarker-evaluable population (BEP). The baseline characteristics (IPI score, cell of origin, and high-risk features), PFS, and OS rates were similar between the ITT and BEP; ctDNA analysis results of the BEP are reported below.
Correlations between ctDNA, clinical factors, and outcomes
Between the pola-R-CHP and R-CHOP arms, there was no significant difference (p = 0.2) in baseline ctDNA levels, with a median MMPM of 356 vs 237, respectively.
- High levels of baseline MMPM were significantly associated with the following baseline clinical factors: a high baseline IPI score, activated B-cell subtype DLBCL, Eastern Cooperative Oncology Group Performance Status, and presence of bulky disease (>7.5cm; p < 0.001).
- Higher baseline MMPM levels (≥ median vs < median MMPM) was associated with a shorter 24-month PFS (71% vs 81%) and 24-month OS rate (86 vs 94%) in both unadjusted and adjusted analyses.
The ctDNA kinetics from baseline to C2D1 were comparable between both arms. In the pooled analysis:
- Patients with a higher LFC from baseline to C2D1 achieved a significantly favorable EOT clinical response compared with those who had a lower LFC (p < 0.001).
- The prognostic value of a ctDNA LFC of 2.0 was validated, with a more favorable PFS and OS benefit seen in the LFC ≥2.0 group compared with the <2.0 group (PFS, 82% vs 65%; OS, 92% vs 86%).
- Patients with ctDNA clearance at C2D1 achieved a higher PFS (90 vs 71%) and OS rate (96% vs 87%) compared with those who did not have ctDNA clearance; this was seen in both the unadjusted and adjusted analyses.
Among the Pola-R-CHP arm,
- a LFC of ≥2.5 was identified as the optimal cut-off point that allowed the greatest risk stratification of patients, resulting in a PFS difference of 21% in high vs low risk groups;
- a favorable PFS difference (21% vs 14%) was observed when compared with a LFC of 2; and
- ctDNA LFC of 2.5 was also prognostic of PFS and OS, independent of key baseline factors.
TP53 was the most common mutation identified in patients with ctDNA at C2D1. Mutational analyses of ctDNA are ongoing.
Conclusion
The follow-up survival analyses of the POLARIX trial were consistent to primary analyses, with a sustained PFS benefit for the pola-R-CHP arm. Overall, the results suggest that analysis of ctDNA has prognostic value; higher baseline ctDNA levels were associated with baseline clinical factors and shorter survival outcomes, with patients achieving an LFC of ≥2.0 and/or ctDNA clearance yielding superior outcomes than those who did not.
The optimal 2.5 LFC cut-off revealed in the pola-R-CHP analysis can form the basis of future ctDNA guided trials. Further studies will assess ctDNA at the later timepoints, understand the impacts of mutational profiles on treatment, and evaluate the prognostic value of ctDNA within quantitative PET-analyses.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



