All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
ASPEN trial: Long-term follow-up of zanubrutinib versus ibrutinib in patients with WM
Zanubrutinib is a covalent Bruton tyrosine kinase inhibitor that has been approved for adult patients with Waldenström Macroglobulinemia (WM) by the European Medical Agency and the U.S. Food and Drug Administration. Although the ASPEN (NCT03053440) trial did not meet its primary end point at a median follow-up of 19.4 months, zanubrutinib did exhibit encouraging safety and efficacy data compared to ibrutinib. The Lymphoma Hub is pleased to summarize the recently published long-term efficacy and safety analyses of zanubrutinib versus ibrutinib from the ASPEN trial.
Study design
This is a phase III, open-label trial of zanubrutinib versus ibrutinib in patients with WM. Full details of the study design were previously summarized on the Lymphoma Hub. Eligible patients:
- had a previous histological diagnosis of WM;
- were unsuitable for standard chemoimmunotherapy for treatment-naïve patients;
- had no previous treatment with BTK inhibitors; and
- met ≥1 criterion for treatment initiation.
Patients with 88-mutant WM were assigned to cohort 1 and those with 88-wild type WM were assigned to cohort 2. The primary endpoint was very good partial response (VGPR) plus complete response (CR) rates. Other endpoints included progression-free survival, overall survival (OS), and safety.
Results
Efficacy
In cohort 1, 99 patients were assigned to ibrutinib and 102 to zanubrutinib and in cohort 2, 28 patients were assigned to zanubrutinib. After 2-year follow-up:
- VGPR and CR rates were higher for patients who received zanubrutinib compared with ibrutinib at all time points (Figure 1).
- Median time to VGPR was shorter in patients treated with zanubrutinib compared with ibrutinib (6.7 versus 16.6 months).
- Fewer progression-free survival and OS events occurred with zanubrutinib versus ibrutinib (19.6% versus 30.3% in cohort 1). OS was comparable between cohort 1 and 2 (87.5% versus 83.9%).
Figure 1. VGPR rates over 5 years*
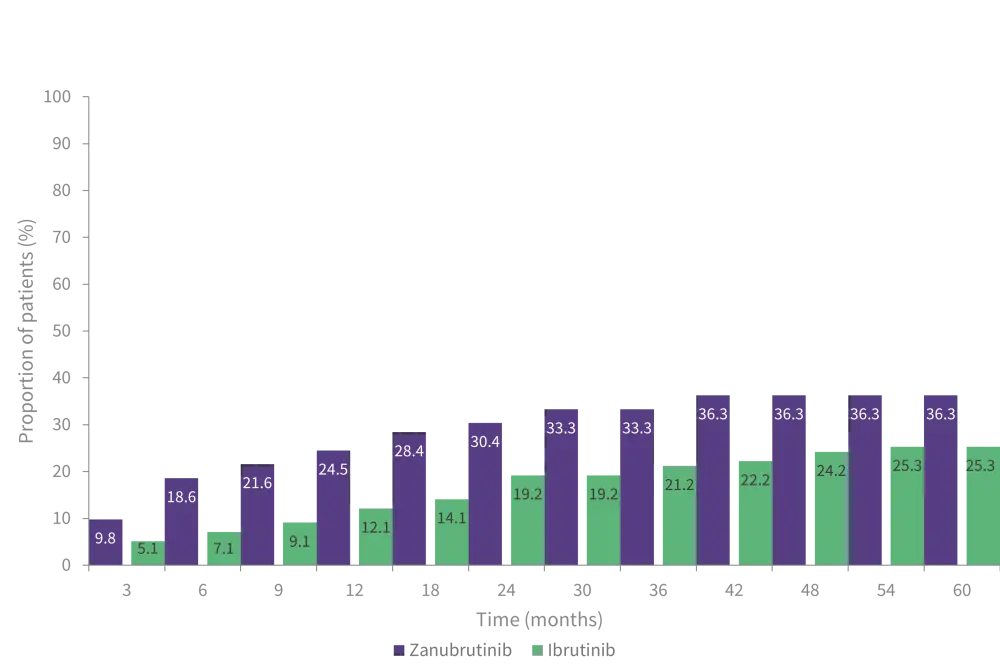
VGPR, very good partial response.
*Adapted from Dimopoulos, et al.1
Safety
- Prevalence of adverse events (AEs) of interest were lower in patients treated with zanubrutinib compared with ibrutinib after 2 years, which is consistent with initial data (Figure 2A and B).
- Although neutropenia was initially reported as more prevalent 0–12 months after treatment in patients treated with zanubrutinib, its prevalence reduced after 2 years.
- After 36 months of treatment, prevalence of infection remained lower in patients receiving zanubrutinib compared with ibrutinib.
- Treatment-emergent AEs leading to discontinuation remained lower in patients receiving zanubrutunib compared with ibrutinib (8.9% versus 20.4%).
- The most common AEs events leading to discontinuation were cardiac disorders, infections, and infestations.
- AEs related to death occurred in eight patients, five with ibrutinib and three with zanubrutinib in cohort 1 and three in cohort 2.
Figure 2. Adverse events of interest reported 0-12 months (A) and >36 months after treatment initiation (B) in patients with zanubrutinib versus ibrutinib*
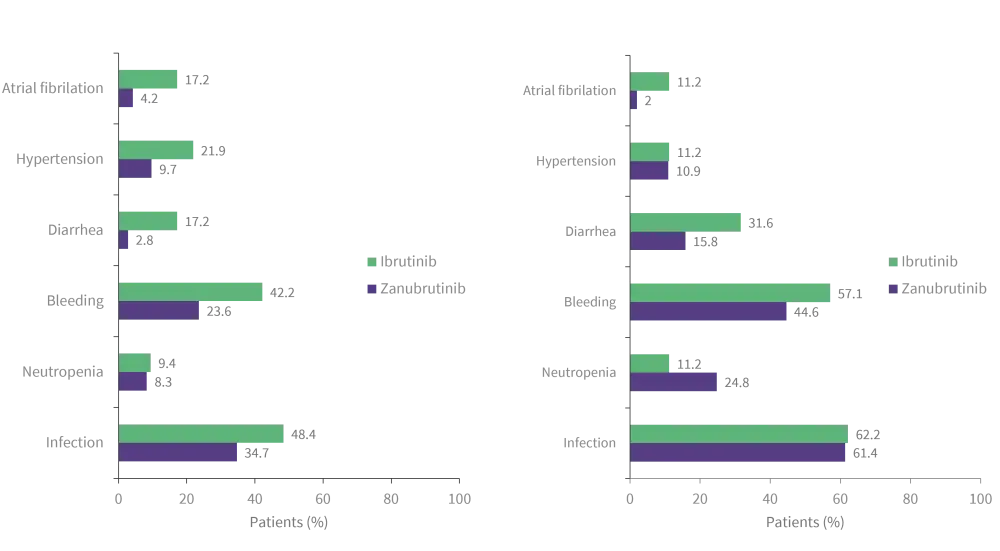
*Adapted from Dimopoulos, et al.1
Conclusion
Extended follow-up results show that treatment with zanubrutinib remained more efficacious than ibrutinib in the long term when comparing VGPR and CR rates. Safety and tolerability of zanubrutinib were comparable to initial results, supporting its use over ibrutinib in patients with WM, regardless of previous treatment or MYD88 mutational status.
‘The International Waldenstrom's Macroglobulinemia Foundation (IWMF) and the Lymphoma Hub are working in collaboration for patients with Waldenstrom's macroglobulinemia. This initiative aims to increase awareness of Waldenstrom's macroglobulinemia among healthcare professionals, patients, caregivers, and the patient advocacy community.
This initiative is funded by Cellectar Biosciences, BeiGene and Eli Lilly. All content is developed independently by SES in collaboration with an expert steering committee; funders are allowed no direct influence on the content of the hub.’
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


