All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
ASPEN trial | Comparison of zanubrutinib vs ibrutinib in WM with MYD88 mutation and zanubrutinib monotherapy in MYD88 wild-type WM
Bruton tyrosine kinase (BTK) inhibition is a desired treatment target in Waldenström’s macroglobulinemia (WM) due to its role in B-cell receptor signaling. Zanubrutinib is under investigation as a potent, selective, irreversible, next-generation BTK inhibitor with the aim of a maximum BTK occupancy and a minimum off-target inhibition of TEC- and EGFR-family kinases.
ASPEN (NCT03053440) is a head-to-head phase III trial comparing zanubrutinib versus ibrutinib, a first-generation BTK inhibitor, in patients with WM who have myeloid differentiation primary response gene 88 (MYD88) mutation. BTK inhibitors have demonstrated significant activity in the presence of MYD88 gene mutation but response rates and progression-free survival (PFS) lower when it is lacking. Investigators also included a second cohort of patients with MYD88 wild-type WM or with unknown mutations to evaluate the efficacy and safety of zanubrutinib monotherapy. Dimopoulos and colleagues presented their results from the two different cohorts during the American Society of Clinical Oncology (ASCO) 2020 Virtual Annual Meeting1 and the Virtual Edition of the 25th European Hematology Association (EHA) Annual Congress.2,3 Here, we provide a summary of the results combined.
The U.S. Food and Drug Administration (FDA) has announced the accelerated approval of zanubrutinib for patients with mantle cell lymphoma, and very recently, the European Medicines Agency (EMA) has accepted marketing authorization application for the treatment of WM based on the results of the ASPEN trial4.
Study Design1–3
Eligibility criteria included:1
- histological diagnosis of WM
- meeting ≥ 1 criterion for treatment initiation*
- being unsuitable for standard chemoimmunotherapy for treatment-naïve patients, and
- no previous treatment with BTK inhibitors
*Clinical indications: recurrent fever, night sweats, weight loss, fatigue, hyperviscosity, symptomatic or bulky lymphadenopathy, symptomatic hepatomegaly, splenomegaly, organomegaly, WM-related peripheral neuropathy. Laboratory indications: symptomatic cryoglobulinemia, cold agglutinin anemia, immune hemolytic anemia or thrombocytopenia, WM-related nephropathy or amyloidosis, hemoglobin ≤ 19 g/dL, platelet count < 100 × 109/L.5
Cohort 1 (comparison cohort) included 201 patients (of which 164 were R/R) with the MYD88 mutation, stratified by C-X-C motif chemokine receptor 4 (CXCR4) status and the number of prior lines of therapy, and randomized in a 1:1 fashion to receive either1,2
- zanubrutinib 160 mg twice daily until progression (n = 102)
- ibrutinib 420 mg once daily until progression (n = 99)
Cohort 2 included 28 patients (of which 23 were R/R) with MYD88 wild-type WM who were treated with zanubrutinib 160 mg twice daily until progression.1,3
The primary objective was to compare the efficacy of zanubrutinib versus ibrutinib based on complete response (CR) or very good partial response (VGPR) in Cohort 1. Secondary objectives were to compare clinical benefit, anti-lymphoma effects, and safety.1,2
Patient characteristics1–3
Cohort 1
- Patient characteristics were well balanced between the two groups1,2
- Median age was 70 years in both treatment arms; the number of patients > 75 years of age was greater in the zanubrutinib arm (see Table 1) 1,2
- The number of patients who received 0, 1–3, or > 3 previous lines of therapy, or who were MYD88L265P/CXCR4WT, were similar across both arms1,2
- More patients had anemia in the zanubrutinib arm1,2
Table 1. Patient and disease characteristics across both arms (intent-to-treat population)1,2
|
CXCR4, C-X-C motif chemokine receptor 4; IPSS WM, International Prognostic Scoring System for Waldenström’s Macroglobulinemia; MYD88, myeloid differentiation primary response gene 88; WT, wild type |
||
|
Characteristic |
Ibrutinib (n = 99) |
Zanubrutinib (n = 102) |
|---|---|---|
|
Age, n (%) > 65 years > 75 years |
70 (70.7) 22 (22.2) |
61 (59.8) 34 (33.3) |
|
Gender, n (%) Male Female |
65 (65.7) 34 (34.3) |
69 (67.6) 33 (32.4) |
|
Previous lines of therapy, n (%) 0 1–3 > 3 |
18 (18.2) 74 (74.7) 7 (7.1) |
19 (18.6) 76 (74.5) 7 (6.9) |
|
Genotype, n (%) MYD88L265P/CXCR4WT MYD88L265P/CXCR4WHIM |
90 (90.9) 8 (8.1) |
91 (89.2) 11 (10.8) |
|
IPSS WM, n (%) Low Intermediate High |
13 (13.1) 42 (42.4) 44 (44.4) |
17 (16.7) 38 (37.3) 47 (46.1) |
|
Hemoglobin ≤ 110 g/L, n (%) |
53 (53.5) |
67 (65.7) |
Cohort 2
- The median age was 72 years; 42.9% of patients were over 75 years old3
- Most patients (n = 23) were relapsed/refractory with at least one previous therapy and had MYD88 wild-type disease (n = 26)3
- The proportion of patients with intermediate- and high-risk disease was 39.3% and 42.9%, respectively3
Results1–3
Cohort 1
By cutoff date of August 31, 20191,2,
- the median follow-up was 19.4 months
- there was no statistically significant difference in CR + VGPR between the two arms (p = 0.09), indicating that the trial did not meet its primary endpoint. As CR is very uncommon in WM, investigators compared VGPR rates in both arms (see Figure 1)
Figure 1. Treatment response across treatment arms1,2
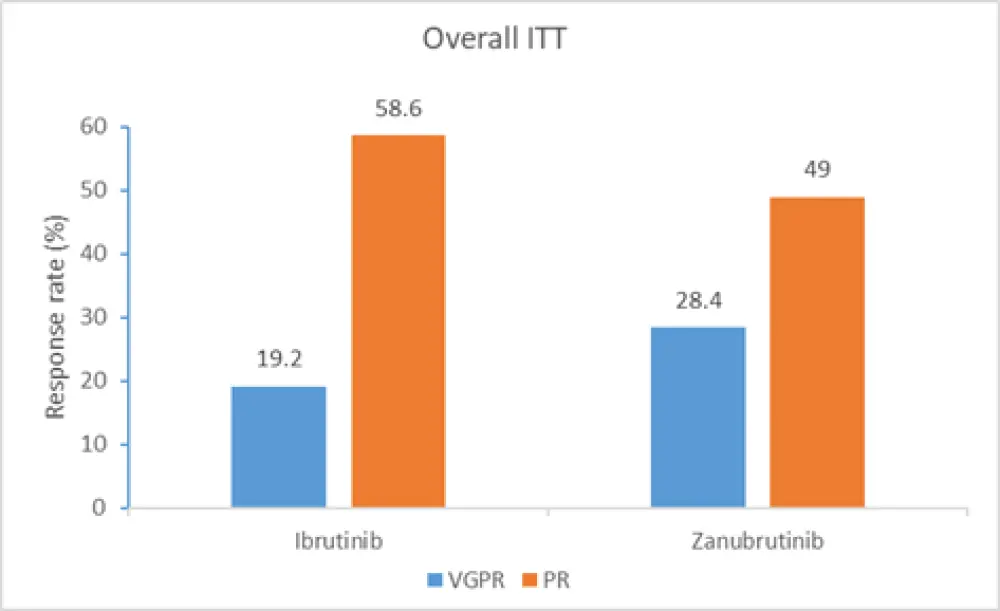
ITT, intent to treat; PR, partial response; VGPR, very good partial response
The difference in VGPR rates assessed by the investigators was statistically significant in the original August 2019 cutoff date (17.2% in the ibrutinib arm vs 28.4% in the zanubrutinib arm; p = 0.04) and at a later cutoff date of January 2020 (18.2% in the ibrutinib arm vs 30.4% in the zanubrutinib arm; p = 0.03). In addition, immunoglobulin M reduction over time was significantly greater in the zanubrutinib arm compared with the ibrutinib arm (p = 0.037).
The PFS and overall survival (OS) probability at 12 months were similar across the zanubrutinib and ibrutinib arms:
- PFS: 89.7% vs 87.2%, respectively
- OS: 97.0% vs 93.9%, respectively
Patients in the zanubrutinib arm with R/R disease tended to have longer PFS and OS at the 12-month time point compared with ibrutinib:
- PFS: 92.4% vs 85.9%, respectively
- OS: 98.8% vs 92.5%, respectively
Safety1,2
The number of patients experiencing at least one adverse event (AE) or serious AEs were similar between the two arms. Key safety findings for Cohort 1 are summarized in Table 2.
Table 2. Adverse event rates across treatment arms1, 2
|
AE, adverse event *p value < 0.05. |
||
|
Category, n (%) |
Ibrutinib (n = 98) |
Zanubrutinib (n = 101) |
|---|---|---|
|
Grade ≥ 3 AEs |
62 (63.3) |
59 (58.4) |
|
Serious AEs |
40 (40.8) |
40 (39.6) |
|
AE leading to death treatment discontinuation dose reduction dose held |
4 (4.1) 9 (9.2) 23 (23.5) 55 (56.1) |
1 (1.0) 4 (4.0) 14 (13.9) 47 (46.5) |
|
≥ 1 treatment-related AE |
84 (85.7) |
80 (79.2) |
|
≥ 1 AE of interest |
81 (82.7) |
86 (85.1) |
|
Most common Grade ≥ 3 AEs (≥ 5%) neutropenia* hypertension* pneumonia* atrial fibrillation/flutter* hemorrhage |
8 (8) 11 (11) 7 (7) 4 (4.1) 8 (8.2) |
16 (16) 6 (6) 1 (1) 0 (0.0) 6 (5.9) |
Updated safety data show a greater difference in atrial fibrillation/flutter rate in favor of zanubrutinib (0% vs 7.1% in the ibrutinib arm), but neutropenia rates increased to 22.8% in the zanubrutinib arm (vs 8.2% in the ibrutinib arm). However, the greater number of neutropenia events did not lead to a higher rate of infections with zanubrutinib as the infection rate was similar between both arms (18.8% for zanubrutinib vs 23.5% for ibrutinib). As patients responded well to both treatments, quality of life improved; but zanubrutinib led to better quality of life scores, which was attributed to better tolerability.
Cohort 2
Median follow-up was 17.9 months. Out of the 28 patients, two patients discontinued treatment due to AEs. None of the patients achieved a complete response. Patients with unknown mutation status achieved partial response.3 Key findings are summarized in Table 3.
Table 3. Best overall response rates in patients with MYD88 wild-type WM3
|
PR, partial response; R/R, relapsed/refractory; SD, stable disease; TN, treatment-naïve; VGPR, very good partial response |
|||
|
Outcome, n (%) |
TN (n = 5) |
R/R (n = 21) |
Overall (n = 26) |
|---|---|---|---|
|
VGPR |
1 (20.0) |
6 (28.6) |
7 (26.9) |
|
PR |
1 (20.0) |
5 (23.8) |
6 (23.1) |
|
Minor response |
2 (40.0) |
6 (28.6) |
8 (30.8) |
|
SD |
1 (20.0) |
3 (14.3) |
4 (15.4) |
|
PD |
0 |
1 (4.8) |
1 (3.8) |
|
Overall response rate |
4 (80.0) |
17 (81.0) |
21 (80.8) |
PFS event-free survival at 12 months was 72.4%.
Safety analysis showed that the most common AEs included diarrhea, anemia, contusion, pyrexia, and upper respiratory tract infection. While one patient experienced atrial fibrillation, two patients experienced major hemorrhage.
Conclusion
The results from the comparison cohort demonstrate that the primary objective of study was not met, with similar rates of CR + VGPR across two arms, but secondary endpoints, including investigator-assessed response rates and immunoglobulin M reduction over time, favored zanubrutinib. PFS and OS rates were also comparable, with both treatments associated with favorable PFS and OS. The safety profile and tolerability of zanubrutinib were considered better based on lower rates of AEs leading to death, treatment discontinuation, or interruptions, and statistically significant lower rates of atrial fibrillation/flutter, hypertension, and pneumonia.
Zanubrutinib demonstrated clinically meaningful antitumor activity in the MYD88 wild-type WM cohort, with high response rates and a favorable safety profile based on low rates of treatment discontinuation.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

