All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Educational theme | Disease management of elderly patients with aggressive B-cell lymphoma in the modern era
Do you know... What is the recommended frontline regimen for patients with aggressive B-cell lymphoma with high-risk features aged ≥80 years?
Introduction
Aggressive B-cell lymphomas, such as diffuse large B-cell lymphoma (DLBCL), are highly prevalent in the elderly population, with a median age at diagnosis of 70 years.1 Although rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is still widely used in this subset, it remains a major clinical challenge with suboptimal treatment outcomes in very elderly patients (aged ≥75 years) with DLBCL when compared with younger patients, be possibly due to an interplay of risk factors such as disease biology, treatment-related toxicities, and clinical factors.1,2 Therefore, risk stratification using prognostic models is an important step to effectively subgroup elderly patients for tailored treatment approaches.3
Novel agents, both as monotherapy and in combination with standard chemotherapy for frontline and relapsed/refractory (R/R) settings, are on the horizon for elderly patients with DLBCL and could aid in optimizing disease management.1,3
Lymphoma Hub recently shared a brief insight on how to improve care for the elderly population. In this review, we will further discuss the strategies for disease management in elderly patients with aggressive B-cell lymphomas, presented by Thieblemont at the Society of Hematologic Oncology (SOHO) 2022 Annual Meeting.4
Prognostic models for baseline risk factors
Identification of ultra-high-risk patients is a crucial step prior to first-line treatment. Over the past few decades since its initial publication in 1993, the International Prognostic Index (IPI) has been developed to allow greater classification of high-risk patients. However, it has been unable to distinguish ultra-high-risk patients and capture biological risk factors.4
Biological heterogeneity has emerged as an important prognostic tool for delineating ultra-risk patients in aggressive B-cell lymphomas. Features such as double- or triple-hit high-grade B-cell lymphoma have been associated with poorer prognostic outcomes. Moreover, clusters C5 and C3 genes within the ABC-like subtype, and C2 gene within the GCB subtype if EZH2 mutated or triple and double-hit HGBCL status, have been found to be unfavorable prognostic factors.4
Recently developed tools, such as baseline total metabolic tumor volume (TMTV), assessed with 18F-fluorodeoxyglucose positron-emission tomography (PET), and circulating tumor DNA (ctDNA), have been identified as good measures of disease burden and survival outcomes, both dependently and independently of IPI.4
Based on the REMARC trial (NCT01122472) in elderly patients aged 60–80 years, TMTV in combination with Eastern Cooperative Oncology Group Performance Status (ECOG PS) was able to more accurately identify the ultra-risk DLBCL population; higher TMTV levels and ECOG PS was associated with inferior survival outcomes.4 The 4-year PFS was 82%, 63%, and 41% in the no risk factor, 1 risk factor (TMTV, >220 or ECOG, ≥ 2), and 2 risk factors (TMTV, >220 and ECOG, ≥2) subgroups, respectively. The 4-year OS rates were 94%, 79% and 59% for no, 1, and 2 risk factors respectively. In general, TMTV and ECOG PS was a better predictor of disease burden prior to first-line treatment compared with IPI. Overall, IPI, TMTV, and ctDNA are strong prognosticators at baseline in high-risk elderly patients with aggressive B-cell lymphoma.4
Management strategies for elderly patients in the frontline setting
For low-risk patients aged ≤60 years with localized disease (Stage I or II) and no age-adjusted IPI risk factors, the current standard treatment is four cycles of R-CHOP every 21 days (R-CHOP21). This regimen has achieved excellent 3-year PFS rates of around 90% in older patients with a good response (interim PET-negative result).4
For patients aged 60–80 years presenting with intermediate or high-risk features, disseminated disease, and at least one age-adjusted IPI risk factor, six cycles of R-CHOP21 are recommended using an interim PET-directed strategy. In accordance with the NCCN guidelines, restaging occurs at cycles 2 or 4 to determine the direction of therapy. Those with a negative interim-PET scan at two or four cycles received the maximum six cycles of R-CHOP. Conversely, those with detected positive interim PET scans at cycles two or four will be considered as having refractory disease and will therefore proceed with second-line treatments.4
In patients aged ≥80 years with high-risk features, six cycles of a dose-modified R-CHOP (R-mini-CHOP) is the established standard treatment.4
Novel treatments in frontline therapy
Novel strategies can be incorporated with frontline therapy, including combinations with R-CHOP chemotherapy backbone, consolidation therapy after chemotherapy, and as a lead-in therapy prior to chemotherapy.1
The phase III POLARIX study (NCT03274492), which recently evaluated the addition of polatuzumab vedotin with R-CHP (pola-R-CHP) vs R-CHOP alone, demonstrated promising results in patients with previously untreated DLBCL. This study, which included patients aged 18–80 years with an IPI of 2–5 and ECOG PS of 0–2, demonstrated a significantly higher 2-year PFS rate in the pola-R-CHP arm compared with R-CHOP alone (76.7% vs 70.2%, respectively); however, the 2-year overall survival rates did not differ significantly between the two arms (88.7% vs 88.6%). Best overall response rate was 95.9% and 94.1% and complete response rates were 86.6 and 82.7% after pola-R-CHP and R-CHOP alone, respectively.4
Another potential novel agent for frontline therapy is the addition of axicabtagene ciloleucel (axi-cel), evaluated in the ZUMA-12 trial (NCT03761056). In this trial, patients had high-risk LBCL, defined as an IPI score of ≥3, and PET-scan positivity, defined as a Deauville score of 4 or 5, following R-chemo treatment. Axi-cel was shown to be efficacious after two cycles of R-CHOP or R-EPOCH, with an overall response rate (ORR) of 85% and complete response (CR) rate of 74%.4
Further strategies include the bispecific antibody epcoritamab, which was assessed in combination with six cycles of R-CHOP in the EPCORE-NHL-2 trial (NCT04663347); previously reported on the Lymphoma Hub. At baseline, 33 patients with DLBCL, a median age of 66 (range, 19–82 years), IPI of 3–5, ECOG PS of 0–2, and double- and triple-hit lymphomas in 20% and 15%, respectively, were included. Epcoritamab yielded an ORR and CR of 100% and 77%, respectively.4
The SMART-START trial (NCT02636322), also reported on the Lymphoma Hub, evaluated another key novel agent being considered in the frontline setting. It studied the efficacy of two cycles of rituximab, lenalidomide, and ibrutinib (RLI) alone prior to the use of standard chemotherapy and six cycles of RLI in combination with R-CHOP or EPOCH in adult patients and those with the ABC-subtype.4 Included patients were aged 29–83 years (median, 63.5 years). After two cycles of RLI alone, the CR and ORR rates were 36.2% and 86%, respectively, and after two cycles RLI plus six cycles of RLI in combination with standard chemotherapy, ORR and CR rates were 100% and 94.5%, respectively.5
There are several novel strategies in combination with R-CHOP that are currently being investigated in the frontline setting in elderly patients with aggressive B-cell lymphomas, such as the use of acalabrutinib with R-CHOP or R-ECHOP regimens (ESCALADE, NCT04529772; 19-C-0116, NCT04002947). Bispecific molecules with R-CHOP, such as mosunetuzumab (GO40515, NCT03677141) are also being analyzed for this patient subset.
Management strategies for elderly patients in the R/R setting
Currently, standard of care (SoC) treatment in the R/R setting involves salvage chemotherapy and allogeneic stem cell transplant (ASCT); however, more than 50% of patients are not eligible for these regimens due to complications caused by high-risk factors such as advanced age and comorbidities.6
Key considerations in second-line treatment include the timing of relapse and whether patients have experienced R/R disease within ≥12 months. Patients with R/R disease after 12 months can be further stratified into eligible vs non-eligible for ASCT, guiding second- and third-line treatment options (Figure 1). Available treatments for ASCT-ineligible patients include multiagent regimens, such as R-gemcitabine and oxaliplatin (R-GemOx), and newer agents such as polatuzumab with bendamustine and rituximab (pola-BR) and tafasitamab plus lenalidomide (tafa-len).4
Figure 1. Treatment algorithm of second- and third-line regimens for elderly patients*
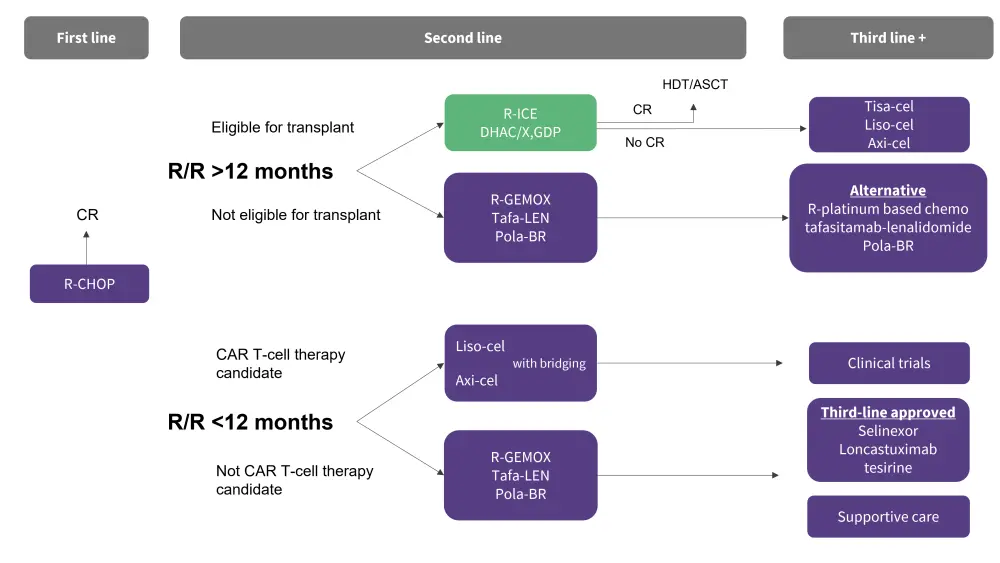
Axi-cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor; CR, complete response; DHAC, dexamethasone, cytarabine, carboplatin; GDP, gemcitabine, dexamethasone, and cisplatin; liso-cel, lisocabtagene-maraleucel; pola-BR, polatuzumab with bendamustine; R-ICE, rituximab, ifosfamide, carboplatin, and etoposide; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-GemOx, R-gemcitabine and oxaliplatin; R/R, relapsed/refractory; tafa-len, tafasitamab plus lenalidomide; tisa-cel, tisagenlecleucel.
*Data adapted from Thieblemont.4
Pola-BR has demonstrated an ORR and CR rate of 45% and 40%, respectively, in treated patients (n = 40).4 Tafa-len achieved an ORR of 60% by independent central review and a CR rate of 43%, as previously reported on the Lymphoma Hub, and was well-tolerated during the L-MIND study.7 These findings resulted in the Food and Drug Administration (FDA) approval of both pola-BR and tafa-len as second-line treatments in patients with LBCL.
For patients who experience R/R disease within 12 months, second-line treatments include lisocabtagene-maraleucel (liso-cel) and axi-cel if they are eligible for CAR T-cell therapy (Figure 1). In the ZUMA-7 trial (NCT03391466) of axi-cel vs SoC (N = 359) in patients aged 21–80 years, axi-cel demonstrated a higher 2-year event free survival rate compared with SoC (40.5% vs 16.3%); the safety profile was also manageable. Following these results, axi-cel received FDA approval in 2022.4
Similarly, the TRANSFORM study (NCT03575351) evaluating liso-cel led to a significantly improved median event-free survival when compared with the SoC group (10.1 months vs 2.3 months; p < 0.0001) and a subsequent FDA approval. In patients with R/R disease <12 months who are not deemed a candidate for CAR T-cell therapy, R-GemOx, POLA-BR, tafa-len, regimens are proposed.4
In third-line treatment, CAR T-cell therapies are recommended with curative intent for patients with R/R >12 months and who relapse post-ASCT. The combined range of ORR and CR rates from the ZUMA-1, JULIET, and TRANSCEND trials evaluating axi-cel, tisa-cel, and liso-cel, was 52–83% and 40–58%, respectively. Alternatives to the R-GemOx, POLA-BR and tafa-len in the third line can include platinum-based chemotherapies. Moreover, supportive care therapies and clinical trials are suggested third-line treatments, with selinexor and loncastuximab tesirine already third-line approved. Selinexor achieved an ORR and CR of 28% and 17%, respectively, in patients with R/R DLBCL (n = 175), including the elderly. Loncastuximab tesirine yielded an ORR and CR of 48% and 24%, respectively, in treated patients (n = 145).4
Bispecific CD20 × CD3 monoclonal antibodies, such as epcoritamab and gloflitamab, have demonstrated excellent response rates in third-line treatment and beyond.4 In elderly patients, a CR rate of 39% was achieved for both treatments, with an ORR of 63% and 51% for epcoritamab (N = 157) and gloflitamab (N = 155), respectively.
Conclusion
In conclusion, wider assessments that integrate disease biology, TMTV, and ctDNA are strong prognosticators of ultra-risk patients with aggressive B-cell lymphomas; guiding appropriate treatment selection. R-CHOP remains the gold-standard in frontline therapy for elderly patients with the use of a PET-directed strategy to adapt treatments. With the introduction of novel strategies, in combination with R-CHOP for frontline settings and as single agents in the R/R setting, we anticipate a positive paradigm shift in disease management of elderly patients with aggressive B-cell lymphomas.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content











