All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Educational theme | Tumor intrinsic resistance mechanisms to CAR T-cell therapy in lymphomas
Chimeric antigen receptor (CAR) T-cell therapies have demonstrated significant efficacy in patients with relapsed/refractory B-cell malignancies following multiple lines of therapy, with various CAR T cell products now U.S. Food and Drug Administration (FDA) approved for the treatment of B-cell acute lymphoblastic leukemia (B-ALL), diffuse large B-cell lymphoma (DLBCL), mantle-cell lymphoma (MCL), and follicular lymphoma (FL).1
Despite this, longer-term survival outcomes (5-year PFS rates of 31% and 43% for patients with DLBCL and FL after tisa-cel (tisagenlecleucel) treatment, respectively)2 show that the majority of patients will experience disease progression or resistance to CAR T-cell therapy. As such, there is a need to elucidate the underlying mechanisms of treatment resistance in lymphoma, which can include CAR T-cell dysfunction, immunosuppressive tumor microenvironment, and tumor-intrinsic factors.1,2
The Lymphoma Hub has previously reported on the key genomic features underlying resistance to CD19-directed CAR T-cells. Here, we focus on the tumor intrinsic mechanisms of resistance to CAR T-cell therapy in patients with lymphomas, discuss novel strategies for managing treatment resistance using data published by Lemoine et al. in Journal of Hematology and Oncology1, and summarize data presented by Ruella at the recent EBMT-EHA 5th European CAR T-cell Meeting.2
Tumor intrinsic mechanisms
Tumor intrinsic mechanisms to CAR T-cell therapies include loss of the target antigen, expression of inhibitory receptors, lack of costimulatory ligands, and resistance to immune killing.1
Loss of target antigen1,2
While the loss of target antigen is well documented after CAR T-cell therapy relapse in B-ALL, similar data in lymphomas is lacking. However, loss of CD19 and CD20 and retention of CD79a has been observed in lymphoma biopsies by immunohistochemistry in tisa-cel treated patients experiencing relapse1,2; a 30% incidence of CD19 loss has also been reported in patients with lymphoma relapsing following axi-cel (axicabtagene ciloleucel) treatment.2
Mechanisms of antigen-negative escape to CD19-directed CAR T-cell therapies, which have been largely studied in B-ALL, include convergence of acquired mutations; alternative splicing of CD19, which generates CD19 isoforms with disruption of the target epitope; and transdifferentiation/lineage switching, including the change from lymphoid to myeloid blast. The basis for other target antigen loss can include selection of pre-existing antigen-negative tumor cells, altered maturation/trafficking affecting target antigen expression, and epitope masking (Figure 1).1,2
Figure 1. Mechanisms of target antigen loss for CAR T-cell resistance*
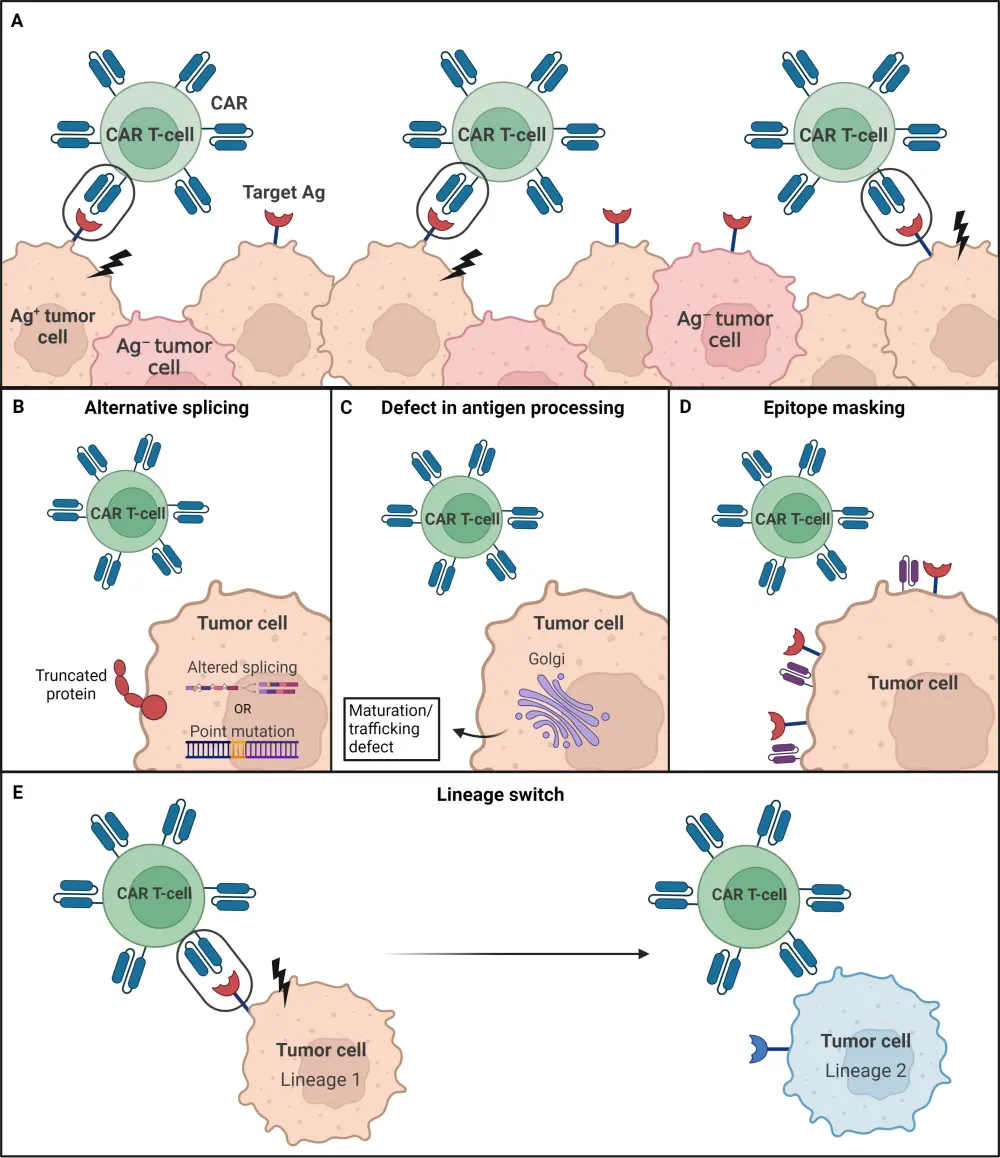
Ag, antigen; CAR T, chimeric antigen receptor T-cell.
*Adapted from Lemoine, et al.1 Created with BioRender.com
Strategies to overcome CD19-negative escape include pooled CAR T cells, dual-targeted CAR T cells, tandem and 4th generation CAR T cells, and combination CD19-directed CAR T cell-therapy (CD19 CAR T-cell) and bispecific antibodies.2
Given the retention of CD79 antigen on lymphoma cells after CAR T-cell relapse, it was considered a suitable therapeutic target for dual CAR T-cell therapy. Among various anti-CD79b CAR T-cell constructs, the CART79#3 structure performed best in murine models when compared with CD19 CAR T-cell candidates. The dual anti-CD19/79b CAR T-cell construct demonstrated tumor cell killing on double-positive blasts compared with the single anti-CD19 and anti-CD79 CAR T-cell therapies. In CD19 and CD79b knockout mice model, this dual CAR T-cell therapy also proved more favorable when compared with single anti-CD19 CAR T-cell and control agents; this is now being explored in clinical trials.2
The combination of CD19 CAR T cells and mosunetuzumab in non-Hodgkin lymphoma is another novel strategy being investigated to overcome resistance in a phase II trial (NCT04889716), with a two-fold concept in mind, 1) targeting both CD19 and CD20 and 2) restimulating CAR T-cell activity by administering mosunetuzumab 30 days after the CAR T-cell infusion.2
Apoptosis2
Apoptosis is a key tumor-intrinsic resistance mechanism of CD19 CAR T cells. A genome-wide CRISPR-based loss-of-function screen, targeting over 20,000 genes in tumor cells at relapse, detected enrichment of pro-apoptotic genes and depletion of anti-apoptotic genes; this indicates the involvement of impaired apoptotic signaling in tumor resistance.
Additionally, a small molecule screen of over 3,000 molecules, alongside CAR T-cells in tumor cells, found that SMAC mimetics (birinapant) and BCL-2 inhibitors (venetoclax) enhanced anti-tumor activity of CAR T-cell therapy. Whilst birinapant proved efficacious within in vitro studies, it was conversely shown to induce apoptosis of CAR T-cells in vivo.
Among a cohort of 87 patients with large B-cell lymphoma treated with CD19 CAR T-cells, those who harbored BCL-2 alterations (either translocation or gain-of-function) yielded lower overall response and overall survival (OS) rates compared to those without BCL2-alterations; this suggests that BCL2 inhibition could be a beneficial treatment strategy for this patients subgroup.
In xenograft mouse models of B-ALL and MCL, venetoclax combined with CD19 CAR T cells initially demonstrated antitumor effects; however, its longer-term effects were worse compared with the CD19 CAR T-cell therapy alone. Peripheral blood analysis showed lower CAR T cell levels after combined venetoclax plus CD19 CAR T cells compared with CD19 CAR T-cell monotherapy treatment, indicating that BCL2-inhibition affects CAR T-cell proliferation and likely activates its cell-induced death.
To optimize the therapeutic use of CAR T cells with venetoclax, the F104L mutation, known to confer resistance to venetoclax in tumor cells, was used for development of a venetoclax-resistant CAR T-cell therapy (CART19-CL-2[F104L]). This construct was shown to resist venetoclax and protect CAR T- cells in vitro and was synergistic when combined with venetoclax in vivo; this resulted in 100% OS. Although there is limited data on this combination in clinical settings, a retrospective analysis of venetoclax as bridging therapy prior to brexu-cel (brexucabtagene autoleucel) infusion in patients with MCL demonstrated a higher complete response (CR) and event-free survival rate compared with patients who did not receive venetoclax-based bridging therapy.
Overexpression of wild-type BCL-2 in CD19 CAR T-cells is another investigative treatment strategy shown to enhance its in vivo antitumor activity, with prolonged persistence and peak expansion of CAR T-cells also observed. Among patient T cells analyzed from apheresis products after CAR T-cell therapy, higher BCL-2 expression was seen among those achieving a complete vs no response; this also correlated with prolonged CAR T-cell persistence and OS.
Expression of inhibitory ligands1
Another tumor intrinsic mechanism of resistance to CAR T-cell therapy can involve expression of inhibitory ligands, programmed death-1 ligand-1 (PD-L1) and programmed death-2 ligand-2 (PD-L2); both may prevent CAR T-cell activation and induce immune resistance. In a ZUMA-1 trial (NCT02348216) analysis, PD-L1 was expressed in 62% (13/21) of patients with DLBCL who progressed after axi-cel therapy.
Strategies to overcome PDL1-mediated resistance include combining CAR T cells with anti-PD1/PD-L1 antibodies, anti-PD1-secreting CAR T-cell therapy, and PD-L1-resistant CAR T-cell therapy. A single-center study, including 12 patients with DLBCL who relapsed after CD19 CAR T-cell therapy, investigated the efficacy of sequentially adding the PD1 inhibitor pembrolizumab; of 11 evaluable patients, one achieved a CR, two a partial response, one achieved stable disease, and seven patients experienced disease progression. An overall response rate of 50% was reported in a small study of patients with DLBCL (n = 15) treated with the PD-L1 inhibitor durvalumab, either 21–28 days after (n = 6) or 1 day prior to (n = 9) CAR T-cell infusion, with only 1/5 patients experiencing disease progression after CR.
Overall, 9/10 patients with relapsed/refractory DLBCL achieved an objective response (six with a CR and 3 with a PR) in a ZUMA-6 trial (NCT02926833) investigation the anti-PD-L1 antibody atezolizumab administered subsequent to CD19 CAR T-cell infusion. Engineered CAR T cells with resistance to PD1-signaling are shown to resist inhibition within preclinical models of B-cell lymphomas; however, clinical data are not yet available.
Lack of costimulatory ligand1
Mutations or loss of expression of CD58, a costimulatory ligand for the CD2 molecule involved in T-cell proliferation, is a tumor intrinsic mechanism which is shown to decrease CAR T-cell activation and cytotoxicity within preclinical models. This accounts for approximately 25% of relapsed cases following CAR T-cell therapy in patients with DLBCL and has been associated with poorer outcomes.
The addition of a CAR construct containing a CD2-signaling domain in the cytoplasmic tail could be a potential novel strategy to overcome resistance. So far, it has increased activity in preclinical models of large B-cell lymphomas, but its clinical activity in patients has not yet been established.
Conclusion
Understanding the mechanisms of tumor intrinsic resistance to CAR T-cell therapies is fundamental for the development of novel strategies. Various strategies are being explored to tackle the loss of target antigen, including pooled and dual CAR T cells that incorporate alternative target antigens and investigative combination therapies such as CAR T cells and bispecific antibodies. Strategies have also been devised to ameliorate the impact of impaired apoptotic pathways, including BCL2-inhibition resistant CAR T-cell therapies and BCL2 overexpression. Moreover, CAR T cell/immunotherapy combinations and re-engineered CAR constructs, such as those with a CD2 signaling domain and anti-PD1 secreting CAR T cells, are being investigated to address CD58 loss and PDL1 overexpression in tumor cells. Future work within this area is likely to further optimize the efficacy and personalized use of CAR T-cell therapy in lymphomas.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


