All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Efficacy of ibrutinib plus venetoclax as first-line treatment in patients with CLL/SLL: Primary analysis from the MRD cohort of CAPTIVATE study
Featured:
Ibrutinib, a Bruton’s tyrosine kinase inhibitor, and venetoclax, a BCL-2 inhibitor, are complementary in their modes of action; preclinical and clinical studies have demonstrated the efficacy of this combination in shrinking lymph nodes and clearing peripheral blood (PB) and bone marrow (BM). Ibrutinib and venetoclax both have been approved in the US and Europe for first-line treatment of chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).
CAPTIVATE (NCT02910583) is a phase II study of ibrutinib plus venetoclax as first-line therapy in patients with CLL/SLL. The Lymphoma Hub has previously reported preliminary results from the minimal residual disease (MRD) and fixed-duration (FD) cohort of the CAPTIVATE study. Here we present the key findings from the primary analysis of the MRD cohort of the CAPTIVATE study published by Wierda et al. in the Journal of Clinical Oncology.1
Study design
This was a multicenter, international, randomized phase II trial comprising two cohorts: MRD and FD. Eligible patients were ≥18 and <70 years of age with previously untreated CLL or SLL, had measurable nodal disease, Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 and active disease requiring treatment per International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria.
- The primary endpoint was 1-year disease-free survival (DFS) rate in patients with undetectable MRD (uMRD).
- One-year DFS was defined as absence of MRD relapse, progression, or death at least 1 year after random assignment.
- Secondary endpoints included
- uMRD rates in PB and BM
- tumor lysis syndrome (TLS) risk category reduction (proportion of patients at high risk for TLS after ibrutinib lead-in vs baseline)
- progression-free survival (PFS)
- investigator-assessed overall response rate (ORR) using 2008 iwCLL criteria
- complete response (CR) rate, including CR with incomplete BM recovery (CRi)
- duration of response (DoR)
- overall survival (OS)
- adverse events (AEs)
Patients in the MRD cohort received treatment in two phases: a pre-randomization phase and an MRD-guided randomization phase. During the pre-randomization phase, patients received a lead-in of oral ibrutinib 420 mg once daily for three cycles followed by 12 cycles of oral ibrutinib 420 mg once daily and oral venetoclax with a target dose of 400 mg once daily (after a 5-week ramp-up and TLS prophylaxis). Each treatment cycle was 28 days. The treatment and randomization plan has been previously reported here.
During the MRD-guided randomization phase, patients completing three cycles of ibrutinib and 12 cycles of ibrutinib plus venetoclax received an additional cycle of ibrutinib plus venetoclax and were assessed for tumor response and MRD status.
- Patients with confirmed uMRD (<1 CLL per cell 10,000 leukocytes, serially over ≥2 assessments ≥3 months apart in both PB and BM) were randomly assigned 1:1 to double-blinded treatment with placebo or ibrutinib until confirmed MRD relapse (≥1 CLL cell per 100 leukocytes confirmed on two separate occasions) or disease progression.
- Patients in the uMRD Not Confirmed population were randomly assigned 1:1 to open label treatment with ibrutinib or continued ibrutinib plus venetoclax until disease progression or unacceptable toxicity (Figure 1).
Figure 1. Treatment schema and patient disposition*
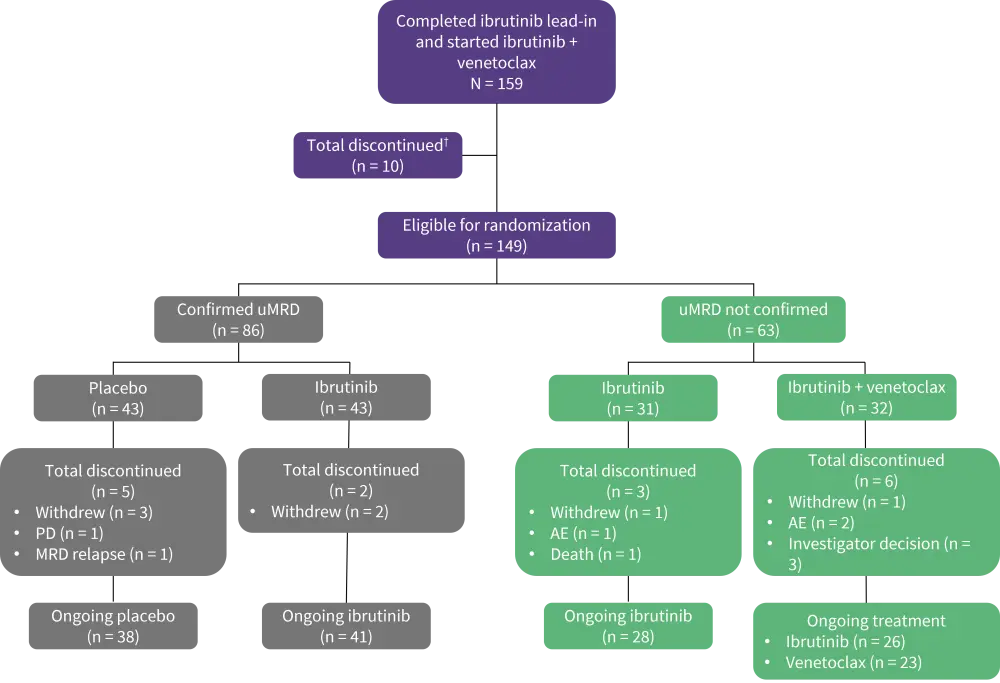
AE, adverse event; MRD, minimal residual disease; PD, progressive disease; uMRD, undetectable minimal residual disease.
*Adapted from Wierda et al.1
†One patient who discontinued venetoclax due to AE (but remained on ibrutinib) was eligible for randomization
Results
Baseline characteristics
A total of 164 patients were enrolled; the median age was 58 years (range, 28–69) and 63% of patients were male. Most patients had features of high-risk disease as summarized in Table 1.
Table 1. Baseline characteristics*
|
ANC, absolute neutrophil count; CLL, chronic lymphocytic leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; IgHV, immunoglobulin heavy chain variable; SLL, small lymphocytic lymphoma. |
|
|
Characteristic, % (unless otherwise stated) |
Total |
|---|---|
|
ECOG PS |
|
|
0 |
64 |
|
1 |
36 |
|
Histology |
|
|
CLL |
96 |
|
SLL |
4 |
|
Rai stage |
|
|
0–II |
68 |
|
III or IV |
32 |
|
Bulky disease |
|
|
≥ 5 cm |
32 |
|
≥10 cm |
3 |
|
Cytopenia at baseline |
|
|
Any |
36 |
|
Hemoglobin ≤ 11 g/dL |
21 |
|
Platelet count ≤ 100 × 109/L |
18 |
|
ANC ≤ 1.5 × 109/L |
9 |
|
Cytogenetics classification† |
|
|
del (17p) |
16 |
|
del (11q) |
17 |
|
Trisomy 12 |
13 |
|
Normal |
15 |
|
del (13q) |
38 |
|
Mutations |
|
|
TP53 |
12 |
|
del (17p) or TP53 |
20 |
|
IgHV mutations status |
|
|
Unmutated |
60 |
|
Mutated |
38 |
|
Complex karyotype‡ |
19 |
Efficacy and safety
Pre-randomization phase
- A total of 148 out of 164 patients completed the pre-randomization treatment phase, with 15 patients who were ineligible for randomization (Figure 1).
- The median treatment duration was 14.7 months (range, 0.5–22.7 months).
- Of the all-treated patients, 75% (123/164) and 68% (112/164) achieved a best MRD response of uMRD in PB and BM, respectively.
- High uMRD rates ranging from 56% to 79% were observed in BM across all subgroups.
- The ORR was 97%, and 46% achieved CR + CRi.
- Among patients who achieved a best response of CR + CRi, 83% achieved uMRD in PB and 80% achieved uMRD in BM.
- Among patients with a best response of PR or nodular PR, 72% achieved uMRD in PB and 61% achieved uMRD in BM.
- After ibrutinib lead-in cycles, a significant reduction in TLS risk was observed with 90% (36/40) of patients with high-risk TLS at baseline moving to medium or low TLS risk categories (Figure 2).
- There was a decrease from 47% at baseline to 18% after ibrutinib lead-in in the proportion of patients with hospitalization indicated for TLS monitoring.
- Overall, 82% of patients initiated venetoclax without hospitalization.
- The most common AEs included diarrhea (71%), nausea (45%), and neutropenia (43%).
- Serious AEs of any grade occurred in 35 patients (21%). No fatal AEs were observed.
Figure 2. TLS risk categories at baseline and after ibrutinib lead-in*
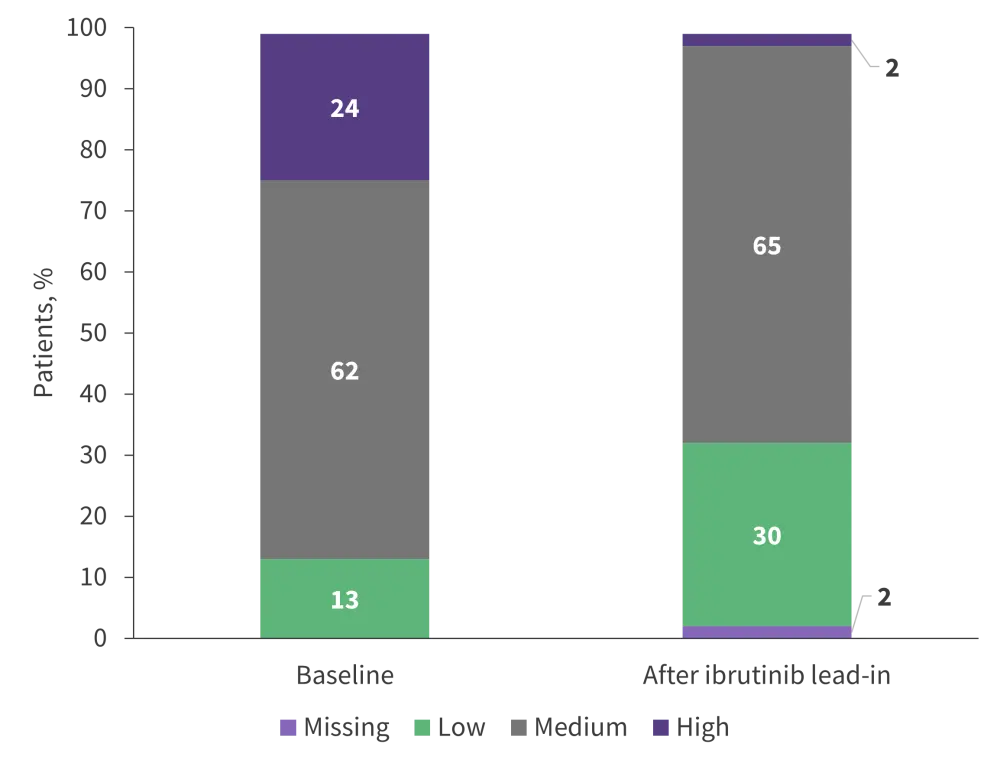
TLS, tumor lysis syndrome.
*Adapted from Wierda et al.1
Confirmed uMRD population
- At a median follow-up of 31.3 months, three DFS events occurred in the placebo arm and no events occurred in the ibrutinib arm.
- There was no statistically significant difference in the DFS rate at 1 year in patients with confirmed uMRD assigned to placebo (95.3%) vs ibrutinib (100%) (p = 0.15).
- Estimated 30-month PFS was 100% vs 95% in the ibrutinib and placebo arms, respectively.
- uMRD in PB and BM was 100% at random assignment, 84% in PB and 81% in BM with placebo, and 77% in both PB and BM with ibrutinib at 12 cycles post-randomization.
- The prevalence of any grade AEs decreased over time after randomization including prevalence of grade ≥ 3 AEs, with a greater reduction in the placebo arm.
- Diarrhea and neutropenia were also less prevalent with placebo vs ibrutinib before and after randomization and decreased over time in both arms.
UMRD Not Confirmed population
- The median treatment duration was 31.2 months and 29.2 months in the ibrutinib and ibrutinib plus venetoclax arms, respectively.
- The estimated 30-month PFS in the uMRD Not Confirmed population was 95% vs 97% in the ibrutinib and ibrutinib plus venetoclax arms, respectively.
- Although the proportion of ibrutinib-treated patients with best response of uMRD remained relatively unchanged (45%) in PB, it improved from 32% to 42% in BM; in patients who received ibrutinib plus venetoclax, best response of uMRD improved from 50% to 69% in PB and from 31% to 66% in BM.
- Grade ≥3 AEs were higher with ibrutinib plus venetoclax than with single agent ibrutinib at 7–12 months post-randomization.
- One Grade 5 AE (sudden cardiac death) occurred in the ibrutinib arm during Cycle 32.
Conclusion
This randomized phase II study demonstrated that high rates of uMRD in both BM and PB can be achieved using ibrutinib plus venetoclax combination as a first-line treatment for patients with CLL/SLL. The 1-year DFS rate of >95% in patients with confirmed uMRD and 30-month PFS rates of ≥95% across MRD-guided randomized treatment arms indicate the potential for FD treatment with this combination. Physicians may be able to use ibrutinib-based therapy (continuous or fixed) in the outpatient setting while considering patient choice. Currently, analyses of FD treatment in a younger population of patients in the CAPTIVATE FD cohort, and in a complementary elderly population in the phase III GLOW study, are ongoing.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Sheetal Bhurke
Sheetal Bhurke