All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Roche and sobi, and supported through educational grants from Bristol Myers Squibb, Incyte and Lilly. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
EHA 2017 | Relapsed/refractory aggressive NHL treatment
On Saturday 24th June, during the 22nd Congress of the European Hematology Association (EHA), a session took place discussing therapy for aggressive relapsed/refractory Non-Hodgkin Lymphoma (NHL). The session was co-chaired by Dolores Caballero from the University Hospital of Salamanca, Salamanca, Spain, and Martin Hutchings from Copenhagen University Hospital, Copenhagen, Denmark. Below are the key highlights from the final two presentations that made up this session, the first three presentations were discussed here.
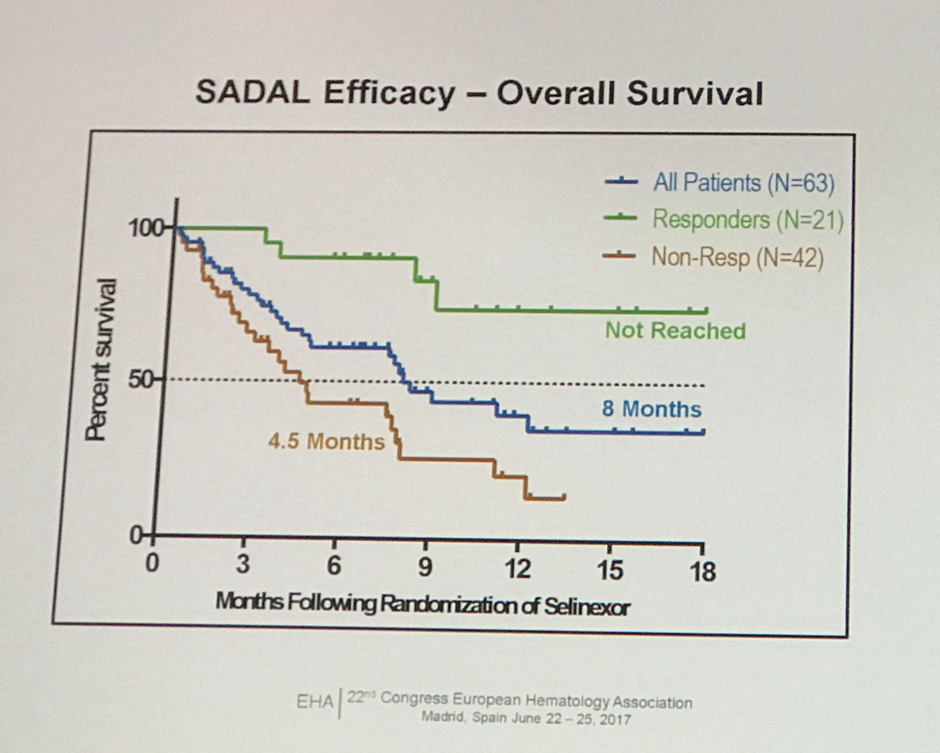
Abstract S469: Selinexor - Phase IIb SADAL study
The fourth abstract in this session was presented by Marie Maerevoet from the Institute Jules Bordet, Brussels, Belgium, on the the results of the interim analysis of the phase IIb SADAL study of single agent selinexor in R/R DLBCL patients (NCT02227251).
Selinexor is a first-in-class inhibitor of exportin 1 (XPO1), a nuclear export protein which is upregulated in certain malignancies including DLBCL. XPO1 transports key proteins out from the nucleus into the cytoplasm including tumour suppressor proteins, in addition to mRNA encoding proteins known to be important in cancer, such as c-Myc and BCL-XL. Selinexor, through inhibiting XPO1, results in the inhibition of cell growth, reduces oncoprotein expression, and blocks NF-kB signalling.
The randomized phase IIb SADAL study compared 60mg vs. 100mg selinexor doses, on a twice per week schedule of a 28-day cycle. The primary endpoint was ORR, and the secondary endpoints were DoR, OS, and safety.
Summary:
- Total number of patients recruited (May 2017) = 90
- Number of patients in the interim analysis = 63
- Selinexor 60 mg = 32 pts
- Selinexor 100 mg = 31pts
- Combined: ORR = 33.3%, CR = 14.3%, PR = 19%
- Selinexor 60 mg: ORR = 34.3%, CR = 12.5%, PR = 21.9%
- Selinexor 100 mg: ORR = 32.2%, CR = 16.1%, PR = 16.1%
- GCB vs. Non-GCB subtype: ORR = 28.1% vs. 38.7%, CR = 12.5% vs. 16.1%
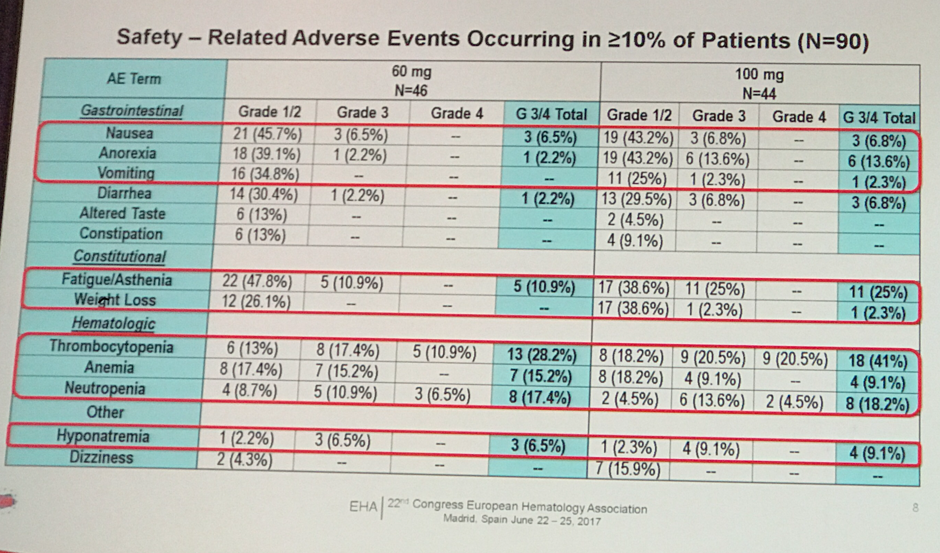
- Most common grade 3 or higher adverse events (AEs) in the 60 mg vs. 100 mg group were: thrombocytopenia (28.2% vs. 41%), fatigue (10.9% vs. 25%) and neutropenia (17.4% vs. 18.2%)
- Safety data showed that 60 mg was better tolerated than 100 mg with lower incidence of grade 3 or higher AEs (shown below)
- It was suggested that AEs can be managed successfully with dose reductions or interruptions in addition to supportive care
In summary, Dr. Maerevoet stated that selinexor demonstrated activity in R/R DLBCL with an ORR of 33.3%, a median DoR of more than 7 months, and a median OS of 8 months (NR in responding patients). However, due to toxicity the 100 mg arm was stopped, but recruitment is ongoing for an additional 90 patients to receive the 60 mg dose. Dr. Maerevoet discussed this data with the Lymphoma Hub in a video after this presentation from EHA 2017.
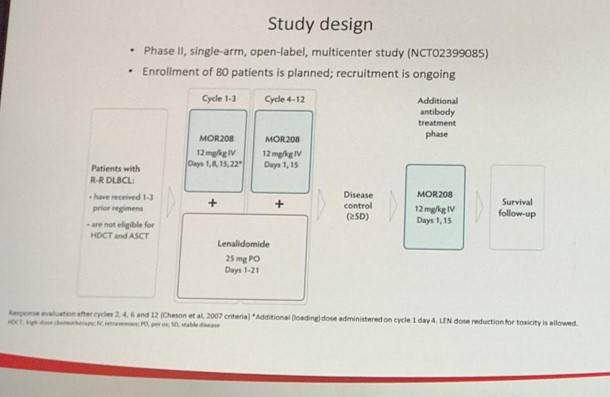
Abstract S470: L-MIND phase II study of MOR208 combined with lenalidomide
The final abstract from this session was presented by Kami Maddocks on the preliminary results of the L-MIND phase II study of the combination of MOR208 and lenalidomide (Len) in R/R DLBCL (NCT02399085). In total 34 patients with a median ages of 73 years qualified for inclusion in the efficacy analysis as of March 2017. MOR208 is an humanized CD19 monoclonal antibody which has previously been shown to have a synergistic antitumor effect when used in combination with lenalidomide in in vivo and in vitro studies.
The primary endpoint was ORR of MO208 + Len, with secondary endpoints including OS, PFS, DoR, and safety of the combination. Inclusion criteria of note included patients must have received at least one prior therapy, but no more than three prior therapies, with at least one being an anti-CD20. Additionally, patients must not have been eligible for stem cell transplant or high-dose chemotherapy, and double/triple-hit DLBCL patients were excluded.
Summary:
- ORR =56%
- CR = 32% (11 pts)
- PR = 24% (8 pts)
- SD = 12% (4 pts)
- PD/Not evaluable = 32% (11 pts)
- Ongoing responses seen in 84% pts (16/19)
- Most common grade 3/4 TEAEs:
- Neutropenia = 43%, pneumonia = 11% thrombocytopenia = 9%
- No reported infusion-related reactions for MOR208
- Lenalidomide dose adjustment was not required in 55% of patients (27% did have dose reductions)
In conclusion, it was stated that the combination of MOR208 with lenalidomide was well tolerated in this preliminary analysis, with 27% of patients requiring a dose reduction in lenalidomide. Furthermore, it was stated that this combination showed ‘encouraging preliminary activity’ in R/R DLBCL patients with a poor prognosis and ineligible for stem cell transplant. During ICML 2017 in Lugano, Professor Gilles Salles spoke about this trial, and Dr. Maddocks also spoke about this trial after her presentation in Madrid.
References
Your opinion matters
In your experience, when do most CRS/ICANS events occur after lisocabtagene maraleucel infusion?

