All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
GLOW trial: A 4-year update on fixed-duration ibrutinib + venetoclax in previously untreated CLL
Do you know... In the 4-year follow-up of the phase III GLOW trial by Niemann et al., which of the following factors was associated with progression-free survival in both the ibrutinib + venetoclax and chlorambucil + obinutuzumab treatment arms?
Bruton kinase inhibitors such as ibrutinib have significantly improved the treatment landscape of chronic lymphocytic leukemia (CLL). Ibrutinib and venetoclax, a B-cell lymphoma 2 inhibitor, are complementary in their mode of action; phase II studies have shown deep, durable responses, and improvements in progression-free survival (PFS), including in patients with high-risk CLL disease.
The Lymphoma Hub has previously reported findings from the GLOW trial. Here, we summarize a recently published article by Niemann et al.1 in The Lancet Oncology on the 4-year follow-up of the phase III GLOW trial (NCT03462719) investigating fixed duration ibrutinib + venetoclax vs chlorambucil + obinutuzumab in patients with previously untreated CLL.
Study design1
GLOW is a randomized, multicenter study conducted across 14 countries in patients with previously untreated CLL. Eligible patients were aged ≥65 years or 18–64 years with comorbidities (cumulative illness rating scale score of >6/creatinine clearance of <70 mL/min), or both and had an Eastern Cooperative Oncology Group performance status of ≤2. Patients were randomized as shown in Figure 1.
The primary endpoint was PFS assessed by an independent review committee, defined as the time from randomization until disease progression or death from any cause.
Secondary endpoints included:
- Measurable residual disease (MRD) negativity rate: the proportion of patients who had undetectable MRD in bone marrow
- Complete response (CR) rate: number of patients who achieved CR or CR with incomplete bone marrow recovery (CRi)
- Overall response rate: number of patients who achieved CR, CRi, or partial response
- Overall survival (OS): time from randomization to death from any cause
- Duration of CR: time from initial CR or CRi until disease progression or death from any cause
- Time to next treatment: time from randomization until the start of any subsequent anticancer therapy and safety
Figure 1. Treatment schema*
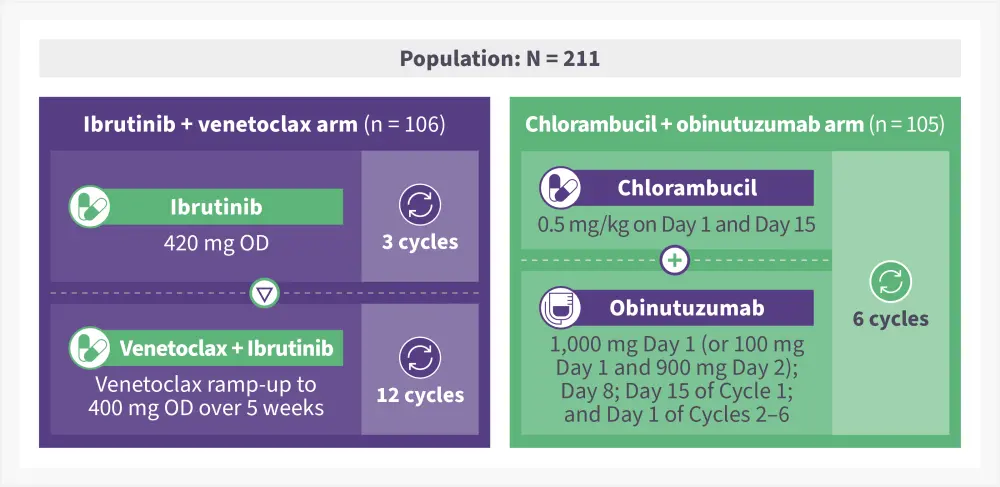
OD, once daily.
*Adapted from Niemann, et al.1
Results1
A total of 211 patients received ibrutinib + venetoclax (n = 106) and chlorambucil + obinutuzumab (n = 105). The baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
Characteristic, % (unless otherwise stated) |
Ibrutinib + venetoclax arm |
Chlorambucil + |
|
|
CIRS, cumulative illness rating scale; CrCL, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; IGHV, immunoglobulin heavy chain variable region gene; IQR, interquartile range; LDH, lactate dehydrogenase. |
|||
|
Median age, years |
71 |
71 |
|
|
Sex |
|
|
|
|
Male |
55.7 |
60.0 |
|
|
ECOG PS 1−2 |
67.0 |
62.9 |
|
|
Median CIRS score, n |
9 |
8 |
|
|
>6† |
69.8 |
58.1 |
|
|
Median CrCL, mL/min |
66.5 |
63.2 |
|
|
Rai stage III–IV |
57.3 |
52.5 |
|
|
Bulky disease ≥5 cm |
39.0 |
36.2 |
|
|
Elevated LDH† |
33.0 |
48.6 |
|
|
IGHV status‡ |
|
|
|
|
Mutated |
30.2 |
33.3 |
|
|
Unmutated |
63.2 |
54.3 |
|
|
Unknown |
6.6 |
12.4 |
|
|
Del(11q) |
18.9 |
17.1 |
|
|
TP53 mutation |
6.6 |
1.9 |
|
Efficacy
At a median follow-up of 46 months:
- The PFS remained significantly higher in the ibrutinib + venetoclax arm vs chlorambucil + obinutuzumab arm, with 29 vs 78 events (p < 0·0001) reported in each arm, respectively (Figure 2).
- The median PFS was not reached in the ibrutinib + venetoclax arm and was 21.7 months in the chlorambucil + obinutuzumab arm.
- Univariate and multivariate analyses revealed a significant association between immunoglobulin heavy chain variable gene (IGHV) mutational status (mutated vs unmutated) and PFS in both treatment arms (p < 0.05).
- In the ibrutinib + venetoclax arm, the 2-year PFS for patients with undetectable (n = 58) and detectable MRD (n = 31), 3 months posttreatment was 93% and 79.6%, respectively.
- In patients with mutated IGHV, the 2-year PFS for those with detectable (n = 14) and undetectable MRD (n = 13), 3 months posttreatment was 92.3% and 100%, respectively.
- In patients with unmutated IGHV, the 2-year PFS for those with detectable (n = 16) and undetectable MRD (n = 40), 3 months posttreatment was 67% and 89.9%, respectively.
- In the chlorambucil + obinutuzumab arm, the 2-year PFS for patients with undetectable (n = 41) and detectable MRD (n = 47), 3 months posttreatment was 66.6% and 18%, respectively.
- Post hoc analysis investigating MRD levels in the ibrutinib + venetoclax group by IGHV mutation status revealed that:
- Undetectable MRD rates were higher in the unmutated IGHV subgroup (52% of 67 participants) vs the mutated IGHV subgroup (31% of 32 participants)
- The MRD kinetics varied in different subgroups, with undetectable MRD rates declining in patients with unmutated IGHV from 60% at 3 months to 36% at 27 months after the end of treatment. Whereas, in patients with mutated IGHV, undetectable MRD rates remained stable in this period (changing from 41% to 44% over this period)
- CR/CRi was 43% vs 12% in the ibrutinib + venetoclax arm and chlorambucil-obinutuzumab arm, respectively.
- CR/CRi at 36 months was 93% in the ibrutinib + venetoclax arm vs 60% in the chlorambucil-obinutuzumab arm.
- Patients in the ibrutinib + venetoclax arm, maintained lymph node clearance, regardless of IGHV mutation or MRD status.
- 8 vs 41 patients (p < 0.0001) required second-line treatment in the ibrutinib + venetoclax arm and chlorambucil + obinutuzumab arm, respectively.
- OS was significantly higher for patients in the ibrutinib + venetoclax vs chlorambucil + obinutuzumab arm (hazard ratio of 0.487; p = 0.021).
- The estimated 42-month OS was 87.5% vs 77.6%, respectively.
Figure 2. 42-month PFS in ibrutinib + venetoclax arm vs chlorambucil + obinutuzumab arm*
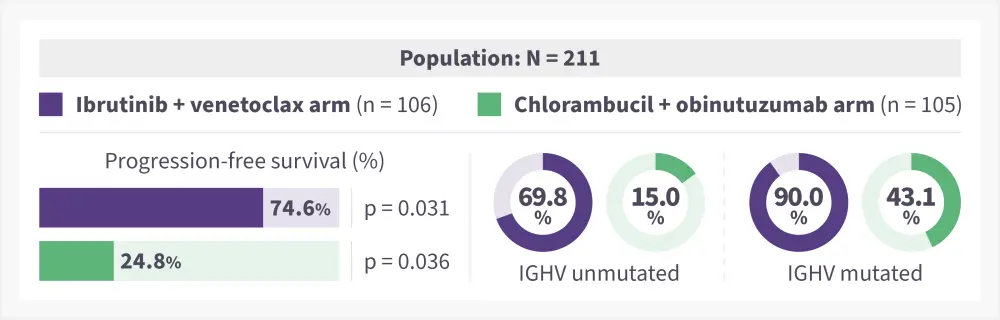
PFS, progression-free survival.
*Adapted from Niemann et al.1
Safety
- There were a greater number of deaths reported in the chlorambucil + obinutuzumab arm vs ibrutinib + venetoclax arm, 30 deaths vs 15 deaths, respectively.
- In the ibrutinib + venetoclax arm, deaths were due to progression (n = 1); treatment-emergent adverse events including cardiac failure, pneumonia, and sinus node dysfunction (n = 7); occurred in remission (n = 6); and occurred during subsequent therapy (n = 1)
- In the chlorambucil + obinutuzumab arm, deaths were due to progressive disease (n = 1); treatment-emergent adverse events including pneumonia (n = 2); occurred in remission (n = 6); occurred after disease progression but prior to subsequent therapy (n = 13); and occurred during subsequent therapy (n = 8).
- There were more infection-related deaths reported in the chlorambucil + obinutuzumab arm compared with ibrutinib + venetoclax arm, 11 vs 4 deaths, respectively.
- A total of 11 patients in the ibrutinib + venetoclax arm and 14 patients in the chlorambucil + obinutuzumab arm experienced a secondary malignancy at longer follow-up.
- In the chlorambucil + obinutuzumab arm, one patient experienced a serious adverse event of myelodysplastic syndrome.
Conclusion
The 4-year follow-up data of the GLOW trial demonstrated that fixed-duration ibrutinib + venetoclax continues to significantly improve PFS and achieve an OS advantage compared with chlorambucil + obinutuzumab in patients with previously untreated CLL. The findings support the clinical use of this combination regimen as a first-line treatment option in patients with CLL.
Supported by an educational grant from Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




