All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Lisocabtagene maraleucel as a second-line treatment for R/R LBCL: PROs from the PILOT study
Results from the phase II PILOT trial (NCT03483103), which were previously covered by the Lymphoma Hub, demonstrated the effectiveness of lisocabtagene maraleucel (liso-cel) as a second-line therapy for patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) not intended for hematopoietic stem cell transplantation.1 Briefly, liso-cel was associated with an overall response rate and complete response rate of 80% and 54%, respectively.1 Results from this trial, in addition to results from the phase III TRANSFORM trial (NCT03575351), led to the approval of liso-cel for patients with R/R LBCL.
Here, we summarize a health-related quality of life (HRQoL) and patient-reported outcomes (PROs) analysis from the PILOT trial, published by Gordon et al.1 in Haematologica.
Study design and patient population1
- The study design and patient characteristics were previously reported by the Lymphoma Hub.
- HRQoL was a secondary endpoint, and was assessed using three PRO instruments:
- European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-30 items (EORTC QLQ-C30)
- Functional Assessment of Cancer Therapy-Lymphoma “Additional Concerns” Subscale (FACT-LymS)
- EuroQoL 5 Dimension 5 Level (EQ-5D-5L).
- PROs were assessed at screening (baseline), before treatment (≤7 days before lymphodepletion), preinfusion on the day of liso-cel infusion (Day 1), after treatment on Days 29, 60, 90, 180, 270, 365, 545, and 730 (end of study), and at disease progression.
- Final PROs were recorded from patients who discontinued the study early.
Key findings1
- Of 61 patients included in the study, 56, 49, 55, and 54 were evaluable for the EORTC QLQ-C30, FACT-LymS, EQ-5D-5L health utility index, and EQ-5D-5L visual analog scale measurements, respectively.
HRQoL at baseline
- When compared with reference age and matched general populations, baseline mean HRQoL scores were slightly worse across most domains, with clinically meaningful worse scores in the EORTC QLQ-C30 fatigue, social functioning, and appetite loss domains.
Within-group changes in HRQoL scores
- Following transient worsening at Day 1 or Day 29, improvements in least squares mean HRQoL scores were generally observed for the primary domains of interest.
- Overall least squares mean changes from baseline through Day 545 are shown in Table 1.
Table 1. Overall least squares mean changes from baseline to Day 545*
|
Domain |
Overall least squares mean change (95% CI) |
p value |
|
CI, confidence interval; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-30 items; EQ-5D-5L, EuroQoL 5 Dimension 5 Level; FACT-Lym, Functional Assessment of Cancer Therapy-Lymphoma; FACT-LymS, FACT-Lym “Additional Concerns” Subscale; GH, global health; HUI, health utility index; QoL, quality of life; VAS, visual analog scale. |
||
|
EORTC QLQ-C30† |
|
|
|
GH/QoL |
2.77 (−0.36 to 5.91) |
0.082 |
|
Physical functioning |
−1.67 (−4.88 to 1.53) |
0.298 |
|
Role functioning |
−3.21 (−8.01 to 1.60) |
0.187 |
|
Cognitive functioning |
−0.55 (−3.50 to 2.41) |
0.711 |
|
Fatigue |
−6.94 (−10.34 to −3.55) |
<0.001 |
|
Pain |
−4.12 (−7.62 to −0.62) |
0.022 |
|
Emotional functioning |
2.47 (−0.36 to 5.30) |
0.086 |
|
Social functioning |
−2.39 (−7.60 to 2.81) |
0.362 |
|
Nausea/vomiting |
1.25 (−0.96 to 3.47) |
0.262 |
|
Dyspnea |
0.99 (−2.61 to 4.59) |
0.585 |
|
Insomnia |
0.76 (−3.77 to 5.29) |
0.740 |
|
Appetite loss |
−5.66 (−9.84 to −1.47) |
0.009 |
|
Constipation |
−1.30 (−4.39 to 1.79) |
0.402 |
|
Diarrhea |
−2.92 (−6.53 to 0.70) |
0.111 |
|
Financial difficulties |
1.27 (−2.72 to 5.26) |
0.526 |
|
FACT-Lym‡ |
|
|
|
FACT-LymS |
4.08 (2.55 to 5.61) |
<0.001 |
|
EQ-5D-5L§ |
|
|
|
EQ-5D-5L HUI |
0.02 (−0.02 to 0.06) |
0.341 |
|
EQ-VAS |
4.35 (1.27 to 7.43) |
0.006 |
Within-patient changes in HRQoL scores
- Most patients showed an improvement or no change in EORTC QLQ-C30 primary domains of interest and FACT-LymS scores from baseline throughout treatment (Figure 1).
Figure 1. Within-patient analysis of changes from baseline for EORTC QLQ-C30 domains of A GH/QoL, B physical functioning, C role functioning, D cognitive functioning, E fatigue, F pain, and for G FACT-LymS*
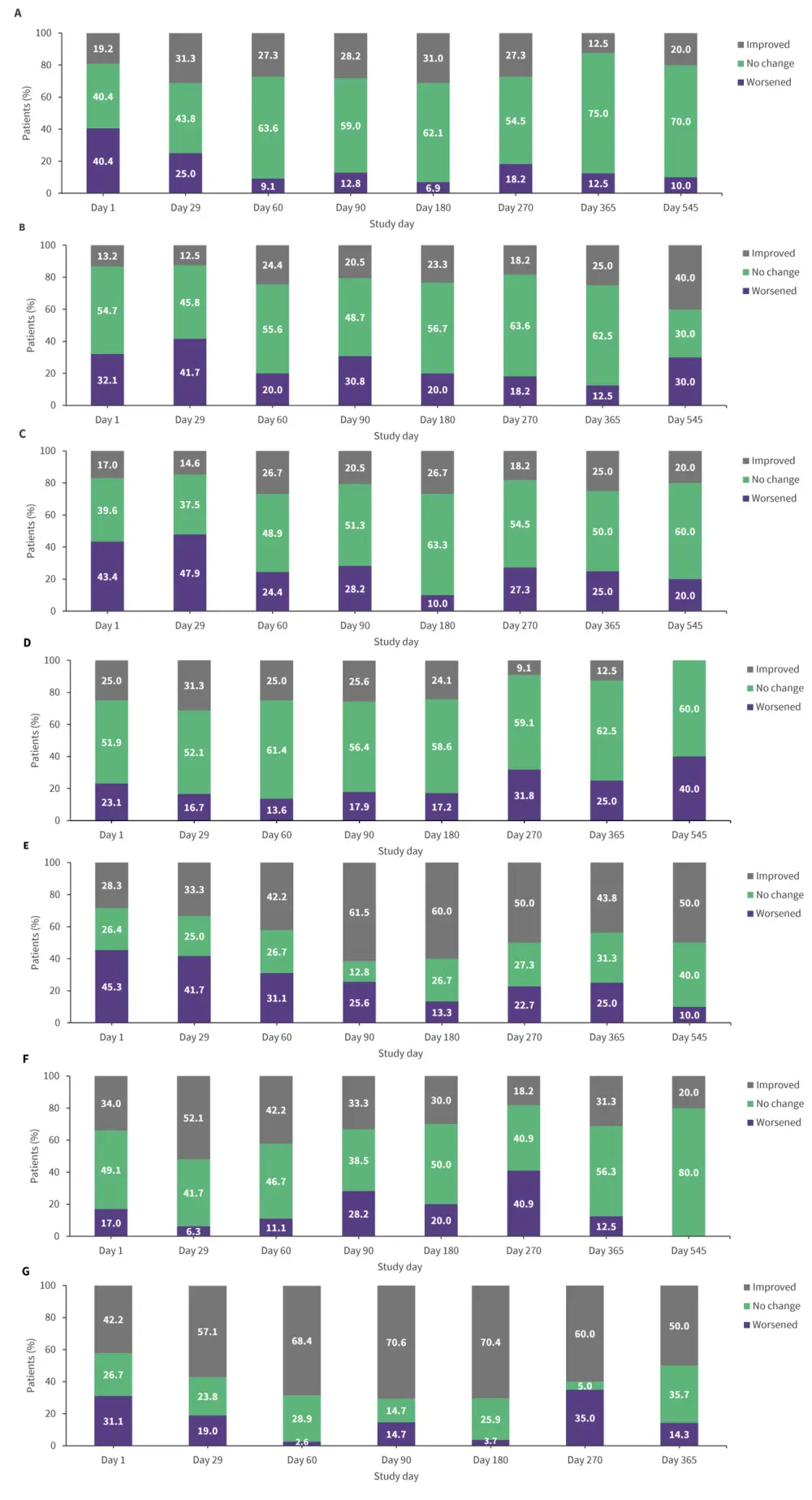
EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-30 items; FACT-LymS, Functional Assessment of Cancer Therapy-Lymphoma “Additional Concerns” Subscale; GH, global health; QoL, quality of life.
*Adapted from Gordon, et al.1
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content







