All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Phase III GLOW trial: Ibrutinib plus venetoclax in previously untreated CLL/SLL
Do you know... In the phase III GLOW clinical trial, how did ibrutinib + venetoclax impact CR/CRi rates vs chlorambucil + obinutuzumab?
During the Lymphoma Hub Steering Committee meeting, Astrid Pavlovsky, Fundaleu, Buenos Aires, AR, chaired a discussion on, Phase III GLOW trial: Ibrutinib plus venetoclax in previously untreated chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL).
Phase III GLOW trial: Ibrutinib plus venetoclax in previously untreated CLL/SLL
Pavlovsky presented and discussed the following topics:
- An overview of current options for first-line treatment of CLL/SLL with Bruton’s tyrosine kinase (BTK) inhibitors
- Ibrutinib + venetoclax: An emerging fixed-duration first-line treatment for CLL/SLL
- GLOW (NCT03462719): Trial design and results
- Long-term outcomes for previously untreated patients with CLL/SLL
Firstly, Pavlovsky provided an overview of selecting first-line treatment in CLL/SLL with fixed-duration and long-term duration approaches.
Pavlovsky went on to discuss clinical data from the phase III GLOW trial (Figure 1). Older patients, and/or those with comorbidities, with previously untreated CLL/SLL were included in the trial.
Figure 1. GLOW: Study design*
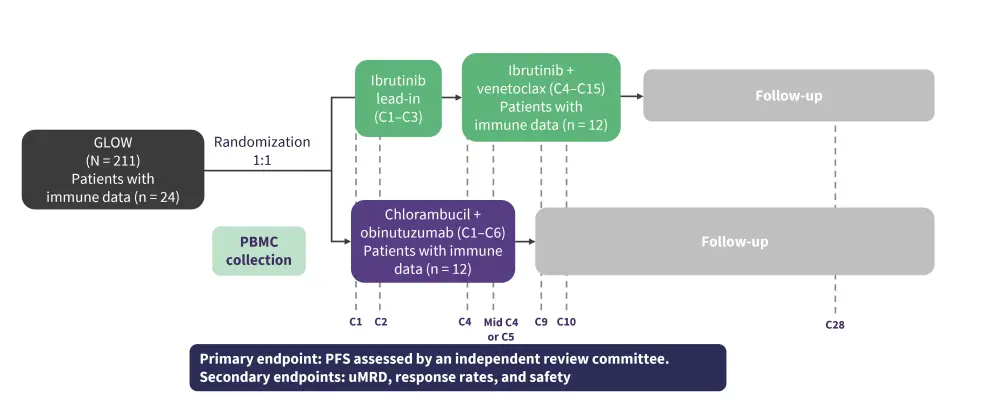
C, cycle; PBMC, peripheral blood mononuclear cell; PFS, progression-free survival; uMRD, undetectable minimal residual disease.
*Adapted from Kater, et al.1
The primary endpoint of PFS was met. With a median follow-up of 27.7 months, there were 22 PFS events for ibrutinib + venetoclax and 67 events for chlorambucil + obinutuzumab. PFS was significantly longer for ibrutinib + venetoclax than for chlorambucil + binutuzumab (HR, 0.216; 95% CI, 0.131−0.357; p = 0.001). Higher response rates were observed in the ibrutinib + venetoclax cohort vs the chlorambucil + obinutuzumab cohort (Figure 2).
Figure 2. Response rates, as assessed by IRC*
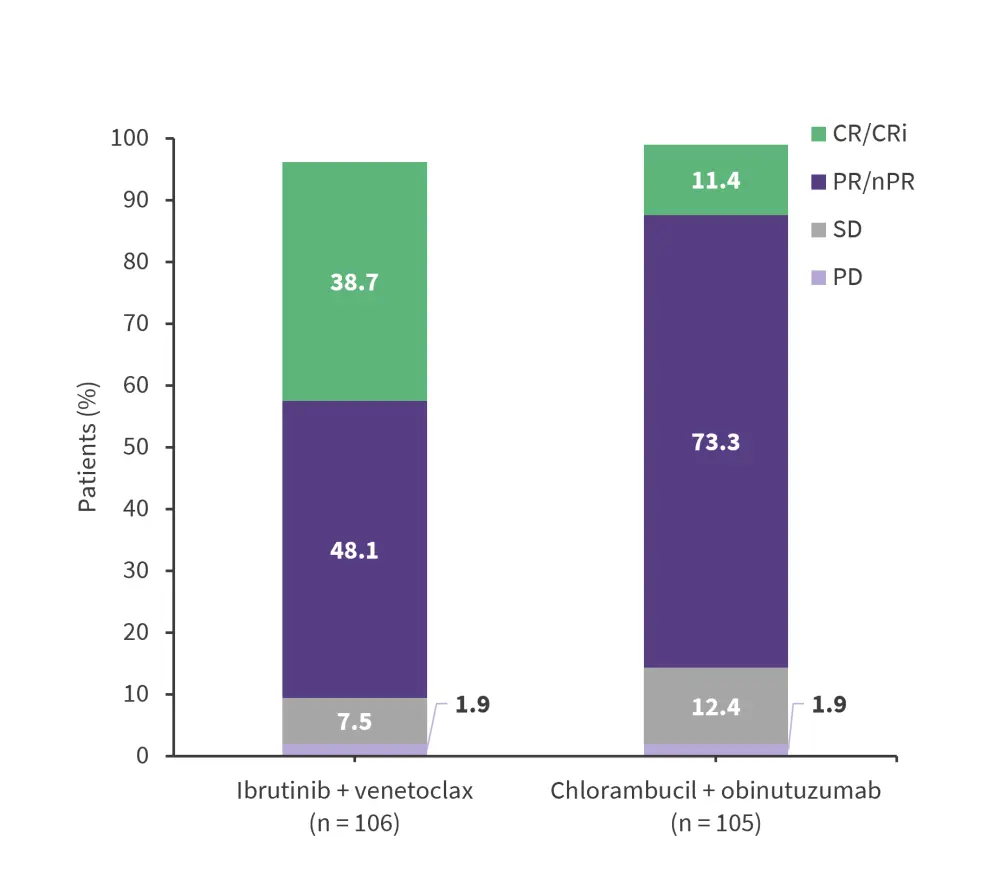
CR, complete response; CRi, CR with incomplete bone marrow recovery; IRC, independent review committee; nPR, nodular partial response; PD, progressive disease; PR, partial response; SD, stable disease.
*Adapted from Kater, et al.1
Furthermore, best undetectable minimal residual disease (uMRD) rate in bone marrow by next-generation sequencing (NGS) at the time of the primary analysis was significantly higher for patients treated with ibrutinib + venetoclax than those treated with chlorambucil + obinutuzumab (55.7% vs 21.0%; p < 0.001). At 3 months after the end of treatment, the proportion of patients in the ibrutinib + venetoclax arm who achieved uMRD in bone marrow was significantly higher vs patients in the chlorambucil + obinutuzumab arm (Figure 3A).
Figure 3. Undetectable MRD rates: A At 3 months after end of treatment. B Peripheral blood MRD (NGS)*
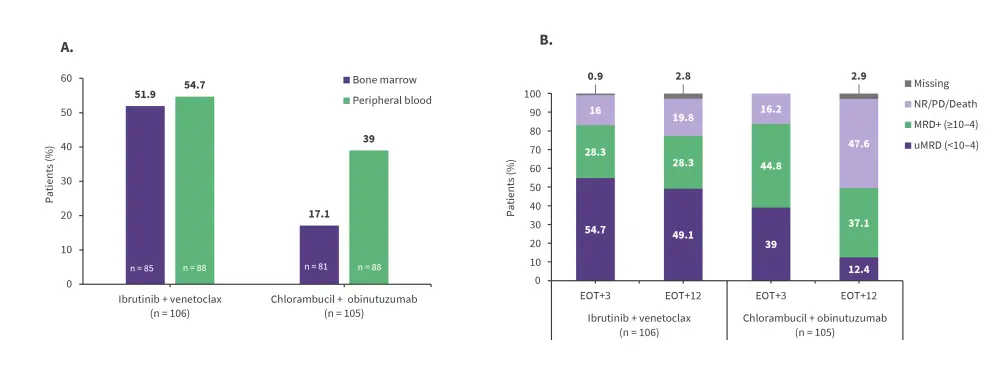
EOT, end of treatment; EOT+3, 3 months after EOT; EOT+12, 12 months after EOT; MRD, minimal residual disease; NGS, next-generation sequencing; NR, non-responder; PD, progressive disease; uMRD, undetectable minimal residual disease.
*Adapted from Kater, et al.1
Pavlovsky then discussed the 4-year follow-up data for the GLOW trial. Fixed-duration ibrutinib + venetoclax continued to significantly improve PFS, with estimated 3.5-year PFS rates of 74.6% for the ibrutinib + venetoclax cohort and 24.8% for the chlorambucil + obinutuzumab cohort. Pavlovsky noted that MRD status had a low impact on PFS. 4-year PFS was significantly longer for patients treated with ibrutinib + venetoclax compared with those receiving chlorambucil + obinutuzumab (HR, 0.214; 95% CI, 0.138−0.334; p < 0.001), and the observed improvement in PFS with ibrutinib + venetoclax was consistent across subgroups such as older patients and those with comorbidities, as well as stratification factors such as IGHV mutational status. The detailed 4-year outcomes were recently published on the Lymphoma Hub.
Furthermore, Pavlovsky discussed the safety data from the GLOW trial. Adverse events of Grade ≥3 occurred for 75.5% and 69.5% of patients treated with ibrutinib + venetoclax and chlorambucil + obinutuzumab, respectively, with neutropenia being most common in both arms at 34.9% and 49.5%, respectively. There were 11 and 12 all-cause deaths in the ibrutinib + venetoclax and chlorambucil + obinutuzumab arms, respectively.
Pavlovsky concluded that, overall, oral, once-daily, fixed-duration ibrutinib + venetoclax provided deeper, longer, and more sustained responses in the GLOW trial vs chlorambucil + obinutuzumab, significantly extending PFS in older patients with previously untreated CLL/SLL and comorbidities.
This presentation was followed by a panel discussion between Pavlovsky, Gilles Salles, Martin Dreyling, Stefano Luminari, Ulrich Jӓger, and Francesc Bosch, covering the following questions:
- What do the phase III GLOW study results mean for clinical practice?
-
How does ibrutinib + venetoclax compare to existing treatments?
Pavlovsky reviewed the GLOW trial results, other fixed-duration regimens, and BTK inhibitor / BCL21 combinations, and the panel debated the practicalities of incorporating fixed-duration ibrutinib + venetoclax as a treatment regimen for previously untreated patients with CLL/SLL. The panel commented that the GLOW trial outcomes reinforce the concept of time-limited therapy for unfit patients. The utility of fixed-duration ibrutinib + venetoclax in older vs younger populations was discussed, and its potential to reduce hospital admissions was noted. The panel emphasized the need for cardiac monitoring with the combination. They also discussed the timing of MRD assessment and the importance of mutational status when deciding between ibrutinib + venetoclax and obinutuzumab + venetoclax. Lastly, it was noted that the ibrutinib + venetoclax regimen is not currently approved in the US.
Supported by an educational grant from Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Ulrich Jäger
Ulrich Jäger Martin Dreyling
Martin Dreyling Stefano Luminari
Stefano Luminari Gilles Salles
Gilles Salles Francesc Bosch
Francesc Bosch Astrid Pavlovsky
Astrid Pavlovsky