All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Phase III inMIND trial: Efficacy and safety of tafasitamab plus lenalidomide and rituximab for R/R FL
Do you know... In the phase III inMIND trial, what was the median duration of response for patients treated with the Tafa-Len-R regimen?
The inMIND trial (NCT04680052) is a double-blind, placebo-controlled, phase III study that evaluated the efficacy and safety of tafasitamab in combination with lenalidomide and rituximab in patients with relapsed/refractory follicular lymphoma or marginal zone lymphoma who have received ≥1 prior lines of therapy.1 The trial was powered to assess progression-free survival in patients with follicular lymphoma only and the planned primary analysis was presented at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition by Sehn et al.1 Here, we summarize the key results as a visual abstract.
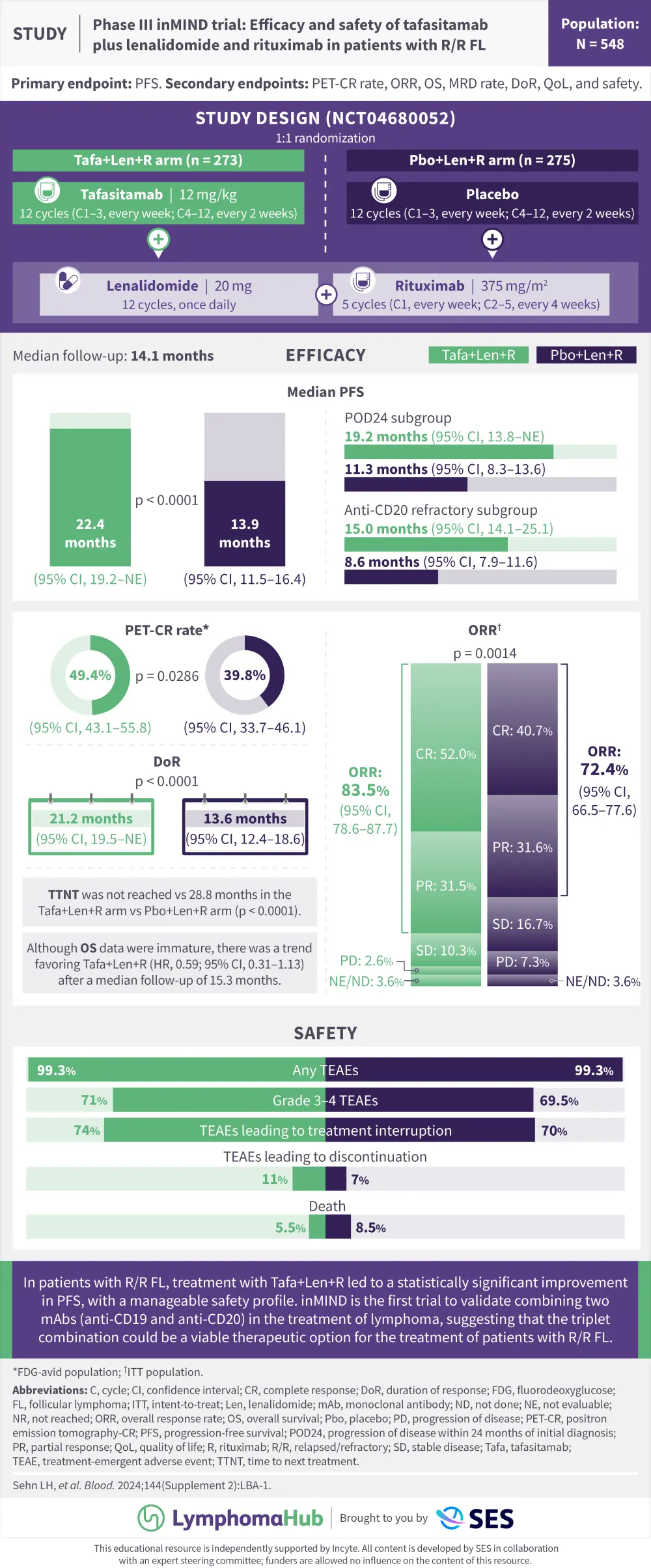
This educational resource is independently supported by Incyte. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

