All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Preparing for CAR T-cell therapy: patient selection, bridging therapies and lymphodepletion
Do you know... In the context of CAR T-cell therapy, which of the following would not be considered when selecting the most appropriate bridging therapy (treatment between apheresis and lymphodepleting chemotherapy)?
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has, in recent years, emerged as a powerful tool in the treatment of certain hematologic cancers. Multiple CAR T-cell products are currently approved by the FDA, with others being evaluated in ongoing clinical trials. An important factor determining the effectiveness of these treatments is whether patients receive them within a reasonable timeframe. In addition, most patients will receive a course of pretreatment lymphodepletion, as well as specific bridging therapy depending upon their individual needs.
Here, we provide a summary of the recently published review article by Amini, et al., in Nature Reviews Clinical Oncology,1 on the many considerations for preparing patients with B-cell non-Hodgkin lymphoma (B-NHL) or acute lymphoblastic leukemia (B-ALL) for CAR T-cell therapy and provide future perspectives on this important treatment area.
CAR T-cell therapy involves apheresis of a patient’s T cells, followed by genetic engineering and clonal expansion. This means that each CAR T-cell therapy is completely unique and tailored to the individual patient. The manufacturing process itself typically takes ≤2 weeks; however, in practical terms, the time between cell apheresis and initial infusion can be much longer. This illustrates the importance of implementing effective interim disease-directed therapy in as timely a manor as possible. Several components for preparing patients with B-NHL or B-ALL for CAR T-cell therapy are outlined below (Figure 1).
Figure 1. Considerations for preparing patients with B-NHL or ALL for CAR T-cell therapy*
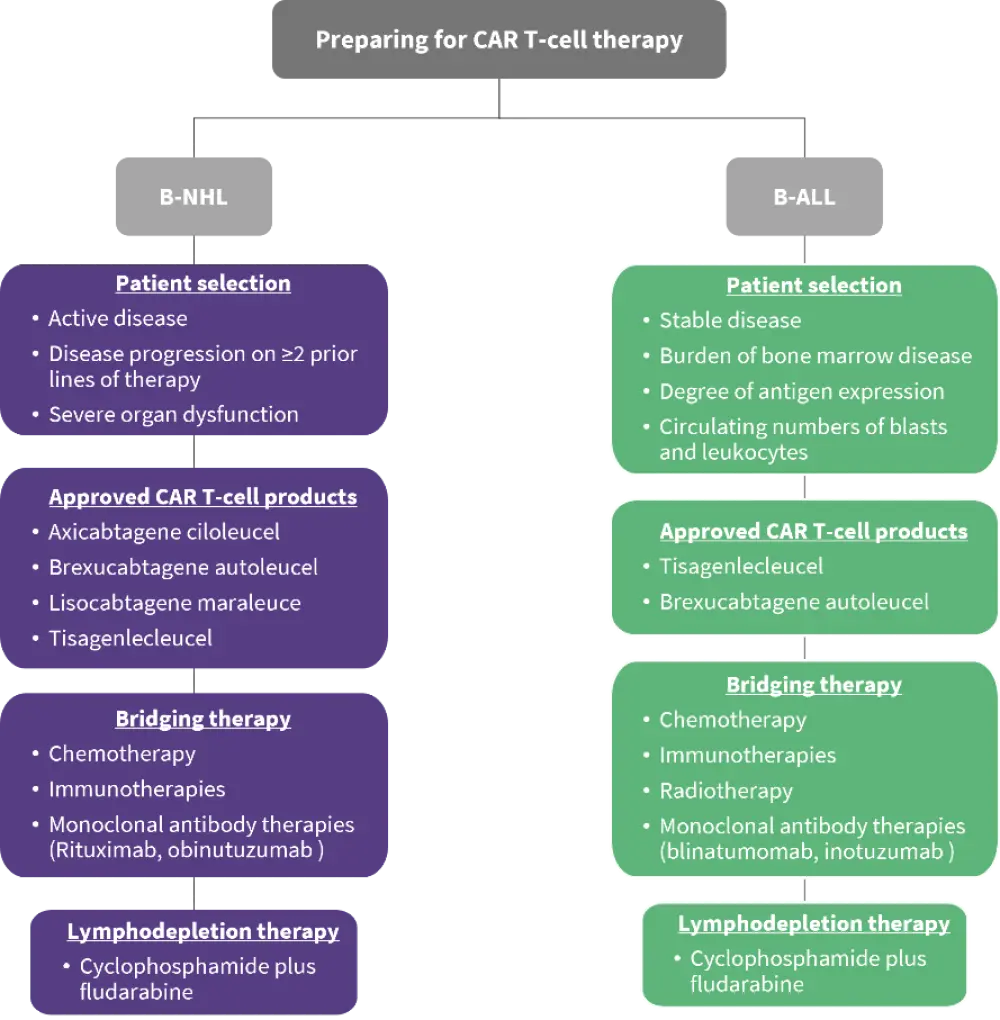
B-ALL, B-cell acute lymphoblastic leukemia; B-NHL, B-cell non-Hodgkin lymphoma; CAR, chimeric antigen receptor.
Data from Amini, et al.1
Patient selection: B-NHL
Four CAR T-cell products are currently approved by the FDA for the treatment of patients with B-NHL: tisagenlecleucel, axicabtagene ciloleucel, lisocabtagene maraleucel, and brexucabtagene autoleucel.1 Patients with B-NHL who are selected for CAR T-cell therapy generally meet the following criteria:
- have disease progression on at least two prior lines of therapy;
- have an active disease state;
- Eastern Oncology Group Performance Status of 0–2; and
- no evidence of severe organ dysfunction, active central nervous system involvement, or acute uncontrolled infections.
While CAR T-cell therapy is currently only approved for heavily pretreated patients, there are signs that patients at an earlier stage of disease progression may also benefit from this therapy, with multiple products currently under investigation as potential early-stage treatments. In addition, a high disease burden prior to CAR T-cell therapy, low platelet count, and high serum lactate dehydrogenase levels have been identified as important risk factors in direct correlation with worse patient outcomes.1
Patient selection: B-ALL
Two CAR T-cell products are currently approved by the FDA for the treatment of relapsed/refractory (R/R) B-ALL: tisagenlecleucel and brexucabtagene autoleucel. The former is approved for the treatment of pediatric and young adult patients with B-ALL, while the latter is approved for the treatment of adult patients with B-ALL. When selecting appropriate patients to receive these therapies, one important consideration is whether the patient is disease stable enough to wait for their specific CAR T-cell therapy to be manufactured. Other important factors include the burden of bone marrow disease, the degree of antigen expression on patients’ leukemic cells, and the circulating numbers of blasts and leukocytes. Once a patient is selected for treatment, the primary consideration is optimization of apheresis timing.
Bridging therapy
In the context of CAR T-cell therapy, bridging therapy refers to treatments between apheresis and lymphodepleting chemotherapy. These treatments aim to slow disease progression during this critical interval period, prior to CAR T-cell infusion.
Three types of bridging therapy have shown effectiveness in trials involving CAR T-cell therapy: immunotherapy, chemotherapy, and radiotherapy. These approaches are often used in combination, which can lead to improved results. Bridging therapy selection is highly variable between patients and is typically specific to both patient and healthcare provider.
Typical considerations for bridging therapy selection:
- predicted time to CAR T-cell infusion
- disease burden
- prior therapy status
- occurrence of specific comorbidities
- risk of adverse-event occurrence (cytopenias and infections)
Bridging therapies for B-NHL
Chemotherapy is the most common bridging therapy for adult patients with R/R large B-cell lymphomas; however, the current data indicate poor outcomes from the use of bridging chemotherapy for the treatment of patients with B-NHL. These observations further underline the importance of carefully identifying patient cohorts who may benefit from systemic bridging chemotherapy. Retrospective studies have indicated that bridging radiotherapy may offer advantages over chemotherapy when used in the context of large B-cell lymphoma.
The monoclonal anti-CD20 antibody therapies rituximab and obinutuzumab are two of the most utilized bridging immunotherapy treatments in patients with B-cell lymphomas. Other monoclonal antibodies including brentuximab, nivolumab, glofitamab, mosunetzumab, and polatuzumab showed favorable outcomes in clinical trials and could be promising bridging therapies in patients awaiting CAR T-cell therapy. Some debate exists as to whether patients receiving bridging immunotherapy should receive CAR T-cells targeting the same antigen due to safety concerns; this may lead to downregulation of the target antigen, potentially rendering subsequent CAR T-cell treatment ineffective. Thus, it is important to ensure the expression of the target epitope before CAR T-cell infusion when immunotherapy targeting the same antigen was administered previously.
Bridging therapies in ALL
Similar to B-NHL, chemotherapy is the most commonly prescribed bridging therapy for patients with ALL. Both high-intensity and low-intensity chemotherapy regimens have been used in both adult and pediatric patients, with low-dose regimens typically resulting in fewer toxicities and infections. Bridging therapy with tyrosine kinase inhibitors is important for patients with Philadelphia chromosome positive B-ALL; however, discontinuation for a wash-out period is necessary before apheresis as the treatment may interfere with CAR T-cell activation.
A number of bridging immunotherapies have shown promise in both adult and pediatric patients with R/R ALL, in particular the monoclonal antibody therapies blinatumomab and inotuzumab ozogamicin which have both demonstrated efficacious responses and a favorable safety profile. However, similar to the bridging therapies for B-NHL, concerns exist regarding the targeting of the same antigen groups that would subsequently be targeted by CAR T-cell treatment. Repeated targeting of the same antigen may lead to antigen escape, indicating a need to monitor antigen expression in this scenario.
Radiotherapy is not a standard of care treatment for ALL outside of the central nervous system. However, this treatment may be useful as a bridging regimen, particularly for patients at risk of severe cytokine release syndrome and reduced response to CAR T-cell therapy due to a high burden of extramedullary disease.
Lymphodepletion
Lymphodepleting regimens are administered prior to CAR T-cell infusion with the goal of improving the expansion and engraftment of the administered CAR T-cells. Lymphodepletion creates a more favorable immune environment for CAR T-cell therapy through a number of mechanisms:
- direct antitumor cytotoxicity
- enhanced homeostatic cytokine production
- reduced immunoregulatory cell numbers
- reduced indoleamine 2,3-dioxygenase expression
Lymphodepletion for B-NHL
All commercial CAR T-cell products currently approved for the treatment of B-NHL are administered after lymphodepletion with cyclophosphamide plus fludarabine. This is one of the most effective lymphodepleting regimens and is received by 98% of patients with R/R B-NHL in clinical settings. However, contraindications to the use of lymphodepleting chemotherapy do exist, particularly for patients with lymphopenia or other cytopenias. In addition, there are conflicting reports as to whether cyclophosphamide or fludarabine preconditioning increases the risk of cytokine release syndrome and neurotoxicities post-CAR T-cell infusion. Taken together, these data underscore the need for continued investigations into these treatments.
Lymphodepletion for ALL
As is the case for patients with B-NHL, lymphodepletion is also an essential pre-treatment for those with ALL, with cyclophosphamide plus fludarabine again being the most widely used regimen. Studies have shown increased complete response rates, improved T-cell persistence, and improved disease-free survival rates in patient groups who received lymphodepletion with cyclophosphamide plus fludarabine, versus those who had not.
Conclusion
CAR T-cell therapies represent a highly effective treatment option for certain patients with hematologic malignancies; however, the most optimal bridging and lymphodepletion strategies for these patients have yet to be fully elucidated. It is hoped that future studies will continue to improve this landmark therapy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

