All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Safety and efficacy of oral azacitidine (CC-486) plus R-CHOP in patients with previously untreated intermediate- to high-risk DLBCL: A phase I study
The combination of rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) has improved outcomes for patients with diffuse large B-cell lymphoma (DLBCL). However, some patient subgroups still have a poor prognosis due to resistance to R-CHOP chemotherapy. Recently, hypomethylating agents (HMAs) have been shown to reprogram the phenotype of cancer cells, generating new vulnerabilities that can be therapeutically explored. Oral azacitidine (oral-AZA; CC-486) is one such HMA that has been approved by both the U.S. Food and Drug Administration (FDA) and the European Commission as a maintenance therapy in adult patients with acute myeloid leukemia (AML). Oral-AZA has the potential to improve R-CHOP by enabling continuous low-dose administration over longer periods of time, potentially boosting epigenetic effects.
Martin et al.1 recently published in Blood a phase I study of CC-486 in combination with R-CHOP in patients with previously untreated DLBCL, Grade 3B follicular lymphoma (FL), or transformed lymphoma (TL).
Study design
This was a phase I, multicenter, open-label, dose-escalation study of CC-486 plus R-CHOP in patients with previously untreated intermediate- to high-risk DLBCL, Grade 3B FL, or TL. Eligible patients were aged 18−80 years, with measurable disease > 1.5 cm, Ann Arbor Stage II−IV disease, an International Prognostic Index (IPI) score of ≥2 or DLBCL double-positive for BCL2 and MYC, and an Eastern Cooperative Oncology Group (ECOG) performance status ≤2.
- The primary aim was to determine the safety and the maximum tolerated dose (MTD) of CC-486 in combination with R-CHOP.
- Secondary aims included characterizing the pharmacokinetics (PK) of CC-486 and preliminary efficacy based on International Working Group (IWG) criteria.
The treatment schedule comprised six 21-day cycles of CC-486 evaluated at escalating doses of 100, 150, 200, or 300 mg. CC-486 was administered orally once daily for 7 days as a priming regimen (Figure 1) prior to initiation of R-CHOP on Day 1 of Cycle 1; thereafter, CC-486 was administered daily for 14 days during Cycles 1−5 of R-CHOP.
Figure 1. Treatment schedule*
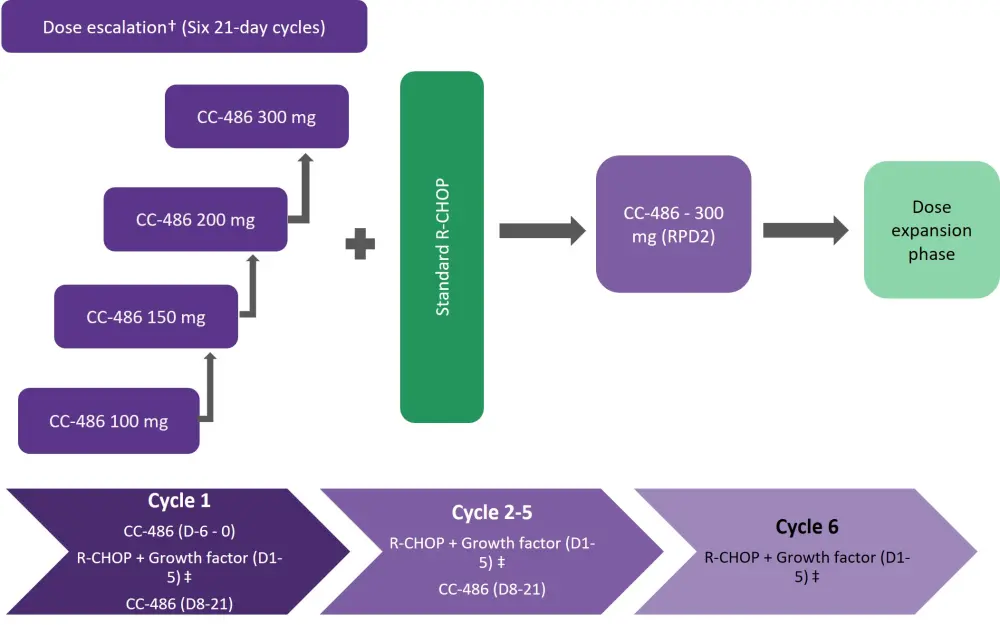
D, day; R-CHOP; rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone; RPD2, recommended phase 2 dose.
*Adapted from Martin et al1
†CC-486 doses of 100, 150, 200, or 300 mg given 7 days before cycle 1 and on days 8-21 of cycles 1-5.
‡Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone; growth factor was administered a minimum of 5 days before the start of CC-486 for pegfilgrastim, and up to 24 hours before the start of CC-486 for lenograstim or filgrastim.
Results
Baseline characteristics
Fifty-nine patients (33 in the dose-escalation phase and 26 in the dose-expansion phase) were enrolled, and all but one patient had DLBCL; the remaining patient had Grade 3B FL. The median age was 66 years (range, 25−80) and 76% of patients were aged ≥60 years; 59% of the patients were male. The baseline characteristics are listed in Table 1.
- A total of 88% of patients treated in both dose-escalation and expansion phases completed all cycles of CC-486, while 92% completed six cycles of R-CHOP.
- The mean relative dose intensity (RDI) of CC-486 was 76% and 82% in patients treated with CC-486 200 and 300 mg doses, respectively. The mean RDI of R-CHOP was similar in patients treated with CC-486 200 mg (96%) and 300 mg (96%).
- The overall mean RDI of CC-486 plus R-CHOP was 90%, with 70% of patients having an RDI > 85%.
Table 1. Baseline characteristics*
|
DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; ESCAL, dose-escalation phase; EXP, expansion phase; GCB, germinal center B cell; IPI, International Prognostic Index; RP2D, recommended phase 2 dose. |
||||
|
Characteristic, % unless otherwise stated |
ESCAL |
EXP |
ESCAL + EXP |
|
|---|---|---|---|---|
|
Overall |
RP2D 300 mg |
RP2D 300 mg |
Overall |
|
|
ECOG PS |
||||
|
0 |
52 |
46 |
53 |
49 |
|
1 |
42 |
50 |
45 |
46 |
|
Ann Arbor disease stage |
||||
|
II |
6 |
8 |
5 |
7 |
|
III |
30 |
31 |
33 |
31 |
|
IV |
64 |
62 |
63 |
63 |
|
Median time between first diagnosis and first dose (range), days |
28 (7−380) |
27 (3−103) |
28 (3−380) |
28 (3−380) |
|
DLBCL† |
27 (9−61) |
25 (3−103) |
24 (3−103) |
26 (3−103) |
|
IPI score |
||||
|
Low/low-intermediate (=2)‡ |
46 |
35 |
45 |
41 |
|
High-intermediate/high (≥3) |
55 |
65 |
55 |
59 |
|
Transformed DLBCL |
15 |
19 |
20 |
17 |
|
Cell of origin§ |
||||
|
GCB |
39 |
46 |
40 |
42 |
|
Non-GCB |
27 |
35 |
35 |
31 |
|
Undetermined |
33 |
19 |
25 |
27 |
|
Overexpression of BCL2 and/or MYCǁ |
66 |
92 |
84 |
78 |
|
Molecular abnormalities |
45 |
57 |
57 |
50 |
Safety
- Treatment-emergent adverse events (TEAEs) were observed in all patients, most commonly nausea (66%), neutropenia (63%), and constipation (58%).
- Grade 3 or 4 TEAEs were mostly hematologic, including neutropenia (63%), febrile neutropenia (25%), anemia (17%), and thrombocytopenia (14%).
- The incidence of TEAEs declined over the course of the study, with most events occurring during Cycles 1 or 2 (98% and 93% of patients, respectively).
- Serious adverse events (SAEs) were observed in 39% of patients, most commonly febrile neutropenia (24%).
- No dose-related toxicity (DLT) was observed at 100 and 150 mg of CC-486. Two patients developed DLT (Grade 4 neutropenia and Grade 4 prolonged neutropenia) at 300 mg.
- Although MTD was not attained, 300 mg was established as the recommended phase 2 dose (RP2D).
Efficacy
- The overall response rate (ORR) was 95%, with 88% of patients attaining complete response (CR) at the 300 mg dose level (Table 2).
- Response rates were similar for patients with IPI scores of 2 and ≥3, with an ORR of 96% and CR in 22 patients (92%) with an IPI of 2, and an ORR of 94% and CR in 30 patients (86%) with an IPI ≥3.
- At a median follow-up of 29 months, the 1- and 2-year progression-free survival (PFS) rates for the overall population were 84% and 79%, respectively (Table 2).
Table 2. Efficacy by treatment phase*
|
CR, complete response; CI, confidence interval; ESCAL, dose-escalation phase; EXP, expansion phase; ORR, overall response rate; PR, partial response; PFS, progression-free survival; RP2D, recommended phase 2 dose; SD, stable disease. |
||||
|
Responses, % unless otherwise stated |
ESCAL |
EXP |
ESCAL + EXP |
|
|---|---|---|---|---|
|
Overall |
RP2D 300 mg |
RP2D 300 mg |
Overall |
|
|
ORR, (95% CI) |
97 (84−100) |
92 (75−99) |
95 (83−99) |
95 (86−99) |
|
CR |
91 |
85 |
88 |
88 |
|
PR |
6 |
8 |
8 |
7 |
|
SD |
3 |
4 |
3 |
3 |
|
Median time to response, months† |
2 |
2 |
2 |
2 |
|
PFS rate at 1 year |
97 |
69 |
78 |
84 |
|
PFS rate at 2 years |
90 |
65 |
72 |
79 |
Pharmacokinetics
- CC-486 plasma exposure demonstrated a dose-proportional increase at the 100 and 300 mg dose levels.
- The terminal half-life of CC-486 alone and in combination with R-CHOP was 0.3−0.6 hours and the plasma clearance ranged from 812−2,335 L/hour.
Conclusion
This prospective study demonstrated that CC-486 plus R-CHOP can be administered safely to patients with previously untreated intermediate- to high-risk DLBCL, Grade 3B FL, and TL. The ORR and CR rates were similar regardless of IPI scores, cell of origin and transformation origin. Future studies are warranted to explore the evaluation of DLTs early in therapy. A prospective phase II/III trial (Southwest Oncology Group/SWOG) evaluating CC-486 plus rituximab and reduced dose of CHOP in elderly patients with DLBCL is currently underway.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

