All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Targeted agents for patients with follicular lymphoma
The virtual Lymphoma Hub Satellite Symposium will take place on Friday, June 18, 2021, at the 16th International Conference on Malignant Lymphoma (ICML). Hosted by Lymphoma Hub Chair Gilles Salles, the symposium will focus on the sequencing of therapies in high-risk relapsed/refractory (R/R) lymphoma and chronic lymphocytic leukemia (CLL). The Lymphoma Hub is proud to introduce the panel of international experts who will be participating: Francesc Bosch, Andrew Davies, Loretta Nastoupil, and Steven Le Gouill.
This article sets the scene for a talk by Loretta Nastoupil outlining the approaches to early-relapsing follicular lymphoma (FL). Why not start by watching the Lymphoma Hub video with Emmanuel Bachy, Hospices Civils de Lyon, Lyon, FR, who outlines how to better treat patients with early-relapsing FL?
How can we better treat patients with early-relapsing follicular lymphoma?
FL is associated with recurrent relapse, necessitating prolonged, intermittent treatment throughout a patient’s lifetime. Established mechanisms behind follicular lymphomagenesis include the B cell receptor (BCR), phosphoinositide 3-kinase (PI3K), and B-cell lymphoma 2 (BCL2) pathways, as well as perturbed epigenetic regulation and antitumor immune response.1 Greater understanding of the driving mechanisms of FL has resulted in the design and approval of a number of targeted agents, which promise to one day replace conventional chemotherapy.
Can we avoid chemotherapy entirely in follicular lymphoma?
Figure 1 highlights the major targeted agents either approved or under evaluation for the treatment of patients with FL. With the exception of lenalidomide, which has demonstrated encouraging response rates as a frontline treatment in combination with anti-CD20 therapy, most agents are reserved for single-agent use as a third-line treatment for FL. Results from clinical trials suggest that PI3K inhibitors and epigenetic regulators are among the most efficacious monotherapies for FL.
Figure 1. Targeted agents for FL.1,2,3*†
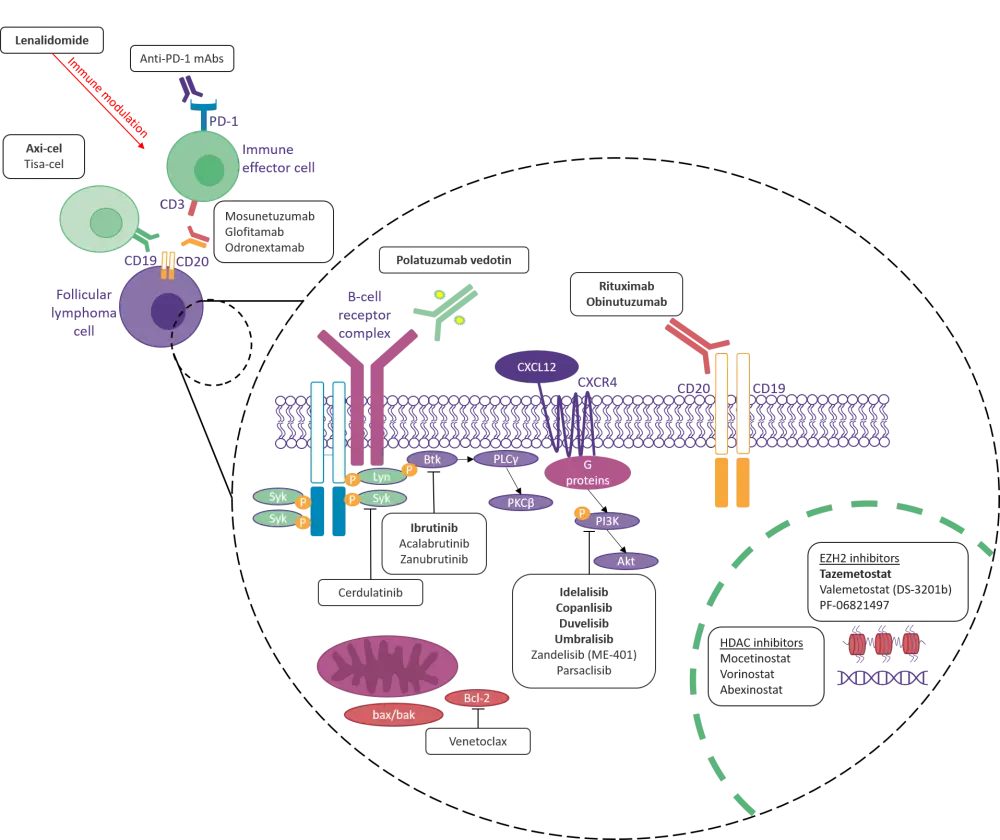
Axi-cel; axicabtagene ciloleucel; CD, cluster of differentiation; CXCL12, C-X-C motif chemokine ligand 12; CXCR4, C-X-C chemokine receptor type 4; EZH2, enhancer of zeste homolog 2; FDA, U.S. Food and Drug Administration; HDAC, histone deacetylase; mAb, monoclonal antibody; PD-1, programmed death receptor 1; PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; Tisa-cel, tisagenlecleucel.
*Bold font indicates agents approved by the FDA.
†Figure adapted from Cheah, Fowler, and Wang.2
Combination therapies
Although therapy with single targeted agents has demonstrated encouraging efficacy in the FL setting, this approach can give rise to drug resistance. Therefore, emerging therapies for FL also include regimens including two or more targeted agents, with the hope to overcome resistance and improve patient outcomes further.1 Data from clinical trials suggest that combination regimens typically achieve deeper, more durable response rates.1 Examples of innovative combinations under evaluation for the treatment of FL are shown in Table 1.
Despite promising response rates, combination regimens have been associated with increased toxicity, and so clinical studies evaluating drug combinations for FL are of utmost importance.
Table 1. Combination regimens under investigation for the treatment of FL.1,4–7
|
Benda, bendamustine; Len, lenalidomide; O, obinutuzumab; ORR, overall response rate; Pola, polatuzumab; R, rituximab; R/R, relapsed/refractory; TN, treatment naïve; Ven; venetoclax. |
||||
|
Regimen |
Trial |
N |
Indication |
ORR, % |
|---|---|---|---|---|
|
O-Pola-Len3 |
46 |
R/R |
76 |
|
|
O-Pola-Ven1 |
71 |
R/R |
87 |
|
|
R-Benda-Ven1 |
51 |
R/R |
84 |
|
|
R-Benda1 |
NCT02187861 |
51 |
R/R |
84 |
|
R-Ven1 |
NCT02187861 |
52 |
R/R |
35 |
|
R-Len1,5 |
147 |
R/R |
78 |
|
|
O-Len1 |
NCT01582776; (GALEN) |
86 |
R/R |
79 |
|
O-Pola6 |
23 |
R/R |
78 |
|
|
Ublituximab, umbralisib, ibrutinib1 |
7 |
R/R |
71 |
|
|
R-acalabrutinib7 |
13 |
R/R |
39 |
|
Conclusion
The treatment landscape for FL has progressed significantly in recent years due to an increased understanding of disease pathology. So far in 2021, two targeted agents have been approved by the U.S. Food and Drug Administration (FDA) in the FL setting—axi-cel and umbralisib—and investigation into novel targeted drug combinations promises to further improve response rates in patients with FL.
Be sure to tune in to the Lymphoma Hub Satellite Symposium for more information on these agents and ongoing trials evaluating novel combinations for the treatment of FL!
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

