All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Targeting unmet needs in CLL
Initially, standard-of-care treatments involving ibrutinib and obinutuzumab were the preferred treatment regimen for the first-line treatment of patients with chronic lymphocytic leukemia (CLL).1 However, follow-up data from phase III clinical trials showed decreased OS over time and a rise in adverse events, such as cardiac toxicity; therefore, ibrutinib is now considered an “other recommended regimen” in the National Comprehensive Cancer Network (NCCN) guidelines.1
Ibrutinib versus BTK-inhibitors as standard-of-care treatment for patients with CLL
Follow-up data from phase III RESONATE II (NCT01722487) and ALLIANCE A041202 (NCT01886872) trials demonstrated a cumulative incidence of cardiac toxicity in patients treated with ibrutinib plus obinutuzumab and ibrutinib plus venetoclax and obinutuzumab; therefore, head-to-head comparator studies were initiated.1
ELEVATE RR (NCT02477696) was a head-to-head trial comparing the safety and efficacy of second-generation Bruton’s tyrosine kinase (BTK) inhibitors (acalabrutinib and zanubrutinib) versus ibrutinib in previously treated patients with CLL. These data demonstrated that BTK inhibitors are well-tolerated and as good as, or in some cases more efficacious than, ibrutinib. Although these studies were in a relapsed/refractory (R/R) setting, it was considered biologically sound to extrapolate this to first-line settings. These data caused a shift in treatment recommendations, with second-generation BTK inhibitors preferred over ibrutinib plus obinutuzumab as the ideal first-line treatment option.
Although there is a standard of care for first-line treatment of patients with CLL, options for patients who are R/R remain unclear.1 Currently, the definition of double refractory is not universally accepted and therefore there is a lack of consistency in reports. Aronson et al. reported promising outcomes with a noncovalent BTK inhibitor and CAR T-cell therapy in heavily pretreated patients.2 Patients should be described as double-refractory only if the disease has progressed after noncovalent BTK inhibitor treatment and not from intolerance or discontinuation for other reasons.1 However, there is variability in outcomes for different patient groups; therefore, a clear definition and treatment path remains to be set for patients with CLL.1
Role of doublet and triplet phase III studies in CLL and the introduction of CAR T-cell therapy
Although BTK inhibitors are considered the preferrable treatment regimen, toxicities are still a concern and patients dislike being on treatment indefinitely.1 Combination treatments, such as doublet and triplet therapy, are being considered due to their finite durations of therapy (Figure 1). However, the addition of further combination treatments to BTK inhibitors needs to be explored. The COVID-19 pandemic highlighted the importance of recognizing infectious complications. In trials of doublet and triplet therapies, patients who died of COVID-19 could have significant toxicities due to the number of agents used; consideration of patient immune dysfunction is essential.
Figure 1. Doublet and triplet phase III studies and their comparator arms in patients with CLL*
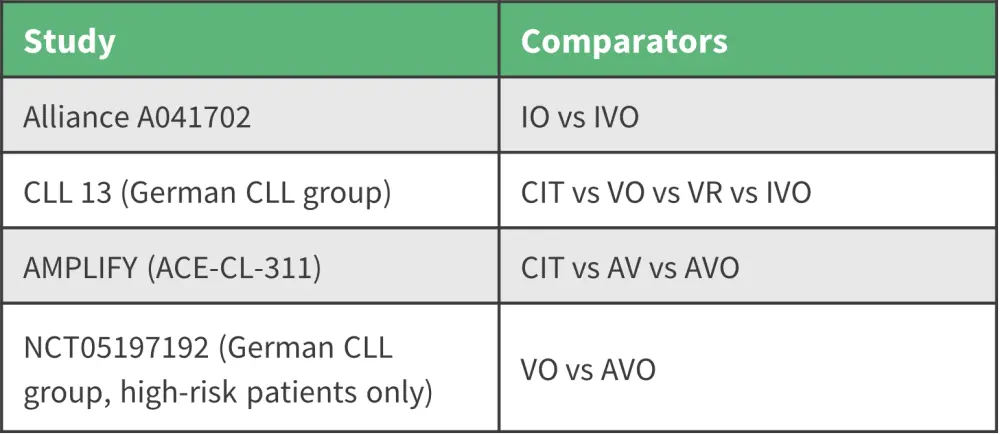
AV, avalabrutinib plus venetoclax; AVO, acalabrutinib plus venetoclax plus obinutuzumab; CIT, chemoimmunotherapy; CLL, chronic lymphocytic leukemia; IO, ibrutinib plus obinutuzumab; IVO, ibrutinib plus venetoclax plus obinutuzumab; VO, venetoclax plus obinutuzumab; VR, venetoclax plus rituximab.
*Adapted from Owen.1
Another treatment option considered for high-risk patients with R/R CLL is CAR T-cell therapy.1 The TRANSCEND CLL 004 (NCT03331198) trial reported consistent median progression-free survival with 20 months of follow-up. Although this involved only a small proportion of the patient cohort, results support this treatment could provide durable remission post-first-line treatment without relapse.1
Richter’s transformation
Richter’s transformation is considered one of the most prevalent unmet needs for patients with CLL,1 with no standard of care for this group and no set treatment plan. Many patients are automatically considered for R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine) therapy when other options, such as venetoclax, atezolizumab and obinutuzumab, should also be considered. The MOLTO (NCT04082897) study, investigating these treatments in patients with Richter’s transformation, showed promising results in patients as first-line therapy. Future phase III studies should investigate R-CHOP versus venetoclax, atezolizumab, and obinutuzumab in patients with Richter’s transformation.1
Key takeaway
The unmet needs addressed in this presentation outline the need for more clinical trials to elucidate the value of each therapy, such as obinutuzumab with BTK inhibitors and the role of “triplets” in patients with CLL (Figure 2).1 The nomenclature of double-refractory CLL has yet to be refined and a clear standard of care is needed for patients with Richter transformation. CAR T-cell therapy is a promising future therapy for high-risk patients with R/R CLL and further phase III trials should explore treatment options beyond R-CHOP.1
Figure 2. NCCN approach to R/R CLL (without del[17p]/TP53 mutation) therapy*
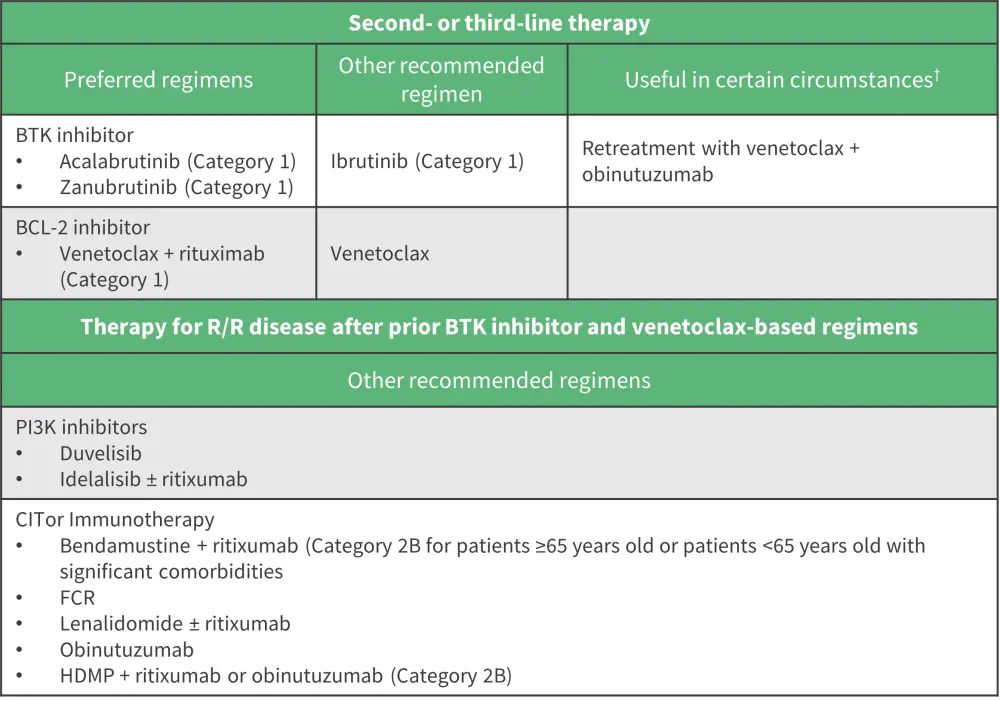
BCL-2, B-cell lymphoma 2 protein; BTK, Bruton’s tyrosine kinase; FCR, fludarabine, cyclophosphamide, rituximab; HDMP, high dose methylprednisolone; PI3K, phosphoinositide 3-kinsae; R/R, relapsed/refractory.
*Adapted from Owen.1
†For relapse after a period of remission if previously used as a first-line therapy.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

