All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
The impact of CRS on CAR T-cell outcomes in patients with aggressive LBCL
Do you know... In the multivariate analyses, which of the following factors was NOT associated with overall survival or progression-free survival in patients with aggressive LBCL treated with anti-CD19 CAR T-cell therapy?
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment paradigm for aggressive large B-cell lymphoma (LBCL); however, it is often associated with adverse events, including the development of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome. There are limited data regarding the impact of CRS development on CAR T-cell therapy efficacy and outcomes.1
The Lymphoma Hub recently reported the impact of bridging therapy on CAR T-cell outcomes in patients with LBCL. Here, we summarize the article published by Bhaskar et al.1 in Blood Advances on the impact of CRS on CAR T-cell outcomes in patients with aggressive LBCL.1
Study design1
This is a retrospective multicenter study that included patients aged ≥18 years with aggressive LBCL who received either axicabtagene ciloleucel or tisagenlecleucel across eight medical centers in the US.
The study outcomes included:
- progression-free survival (PFS), defined as time from CAR T-cell infusion until earliest marker of progression including disease progression, initiation of subsequent anticancer therapy, or death;
- overall survival (OS), defined as the time from CAR T-cell infusion to death from any cause; and
- complete response and overall response rates.
Results1
Of the 351 patients who received CAR T cells, 74.4% developed CRS and the remaining 25.5% did not; 57.6% received axicabtagene ciloleucel and 42.5% received tisagenlecleucel. Baseline characteristics in patients with versus without CRS are summarized in Table 1.
Table 1. Baseline characteristics*
|
axi-cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; IPI, international prognostic index; LDH, lactate dehydrogenase; tisa-cel, tisagenlecleucel; ULN, upper limit of normal. |
|||
|
Characteristic, % (unless otherwise stated) |
Patients with CRS |
Patients without CRS |
p value |
|---|---|---|---|
|
Median age (range), years |
61 (18–88) |
65 (37–85) |
0.005 |
|
Sex |
|
|
0.895 |
|
Male |
66 |
66.7 |
|
|
Female |
34 |
33.3 |
|
|
Stage |
|
|
0.893 |
|
I–II |
19.5 |
18.9 |
|
|
III or IV |
80.5 |
81.1 |
|
|
IPI score |
|
|
0.226 |
|
1–2 |
46.6 |
54.5 |
|
|
≥3 |
53.4 |
45.5 |
|
|
Disease status |
|
|
0.14 |
|
Primary refractory |
37.6 |
21.8 |
|
|
Refractory |
33.9 |
34.6 |
|
|
Relapsed |
28.6 |
43.6 |
|
|
Bulky disease |
|
|
0.330 |
|
Yes |
18.4 |
13.5 |
|
|
No |
81.6 |
86.5 |
|
|
CAR T-cell product |
|
|
<0.001 |
|
Axi-cel |
69.7 |
22.2 |
<0.001 |
|
Tisa-cel |
30.3 |
77.8 |
|
|
Peak ferritin, >5000 |
|
|
<0.001 |
|
Yes |
17.8 |
1.3 |
|
|
No |
82.2 |
98.7 |
|
|
LDH>ULN |
|
|
<0.001 |
|
Yes |
62 |
36.9 |
|
|
No |
32 |
63.1 |
|
|
Received steroids |
|
|
<0.001 |
|
Yes |
46.2 |
4.8 |
|
|
No |
53.8 |
95.1 |
|
|
Received tocilizumab |
|
|
<0.001 |
|
Yes |
59.5 |
12.4 |
|
|
No |
40.5 |
87.6 |
|
|
Received bridging therapy |
|
|
0.227 |
|
Yes |
65.3 |
72.4 |
|
|
No |
34.6 |
27.6 |
|
Survival outcomes
At a median follow-up of 30 months, there were no significant differences in the PFS or OS between patients who developed CRS and those who did not.
- The median PFS for with vs without CRS was 5.89 and 5.99 months, respectively (p = 0.99).
- The median OS for with vs without CRS was 17.5 months and 26.6, respectively (p = 0.16).
- Univariate analyses showed that the CRS grade did not influence PFS or OS.
Multivariate analyses revealed that development of CRS, disease stage (Stage III-IV vs Stage I–II), disease status, or receipt of steroid treatments did not significantly impact PFS or OS; however, a peak ferritin level of >5000 within 28 days following CAR T-cell infusion, and lactate dehydrogenase before the start of lymphodepleting chemotherapy greater than the institutional upper limit of normal was significantly associated with a worse PFS or OS (p < 0.01). Moreover, the presence of bulky disease was significantly associated with a worse OS (p = 0.005), and receipt of bridging therapy was associated with a shorter PFS (p = 0.011).
Response rates and cytopenia analysis
At 30 days post CAR T-cell infusion, there was no difference in objective response rate (p = 0.081) and complete response rate (p = 0.155) between patients who developed CRS and those who did not (Figure 1), or across CRS severity (Figure 2). Further to this, the development of CRS within 28 days was significantly associated with cytopenia at Day 30 (p = 0.001); however, no association between CRS and cytopenia was observed at later time points.
Figure 1. Response rates in patients with vs without CRS*
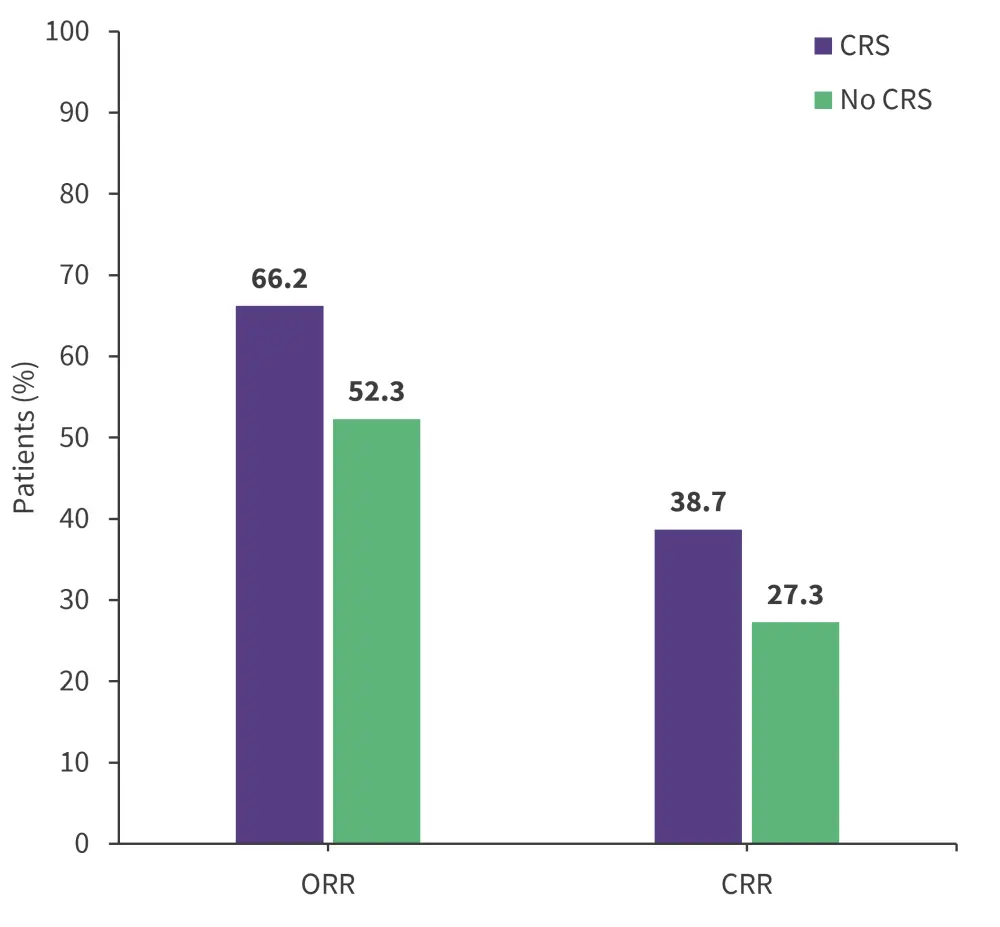
CRR, complete response rate; CRS, cytokine release syndrome; ORR, overall response rate.
*Data from Bhaskar, et al.1
Figure 2. Response rates in patients with no CRS, Grade 1–2 CRS, and Grade ≥3 CRS*
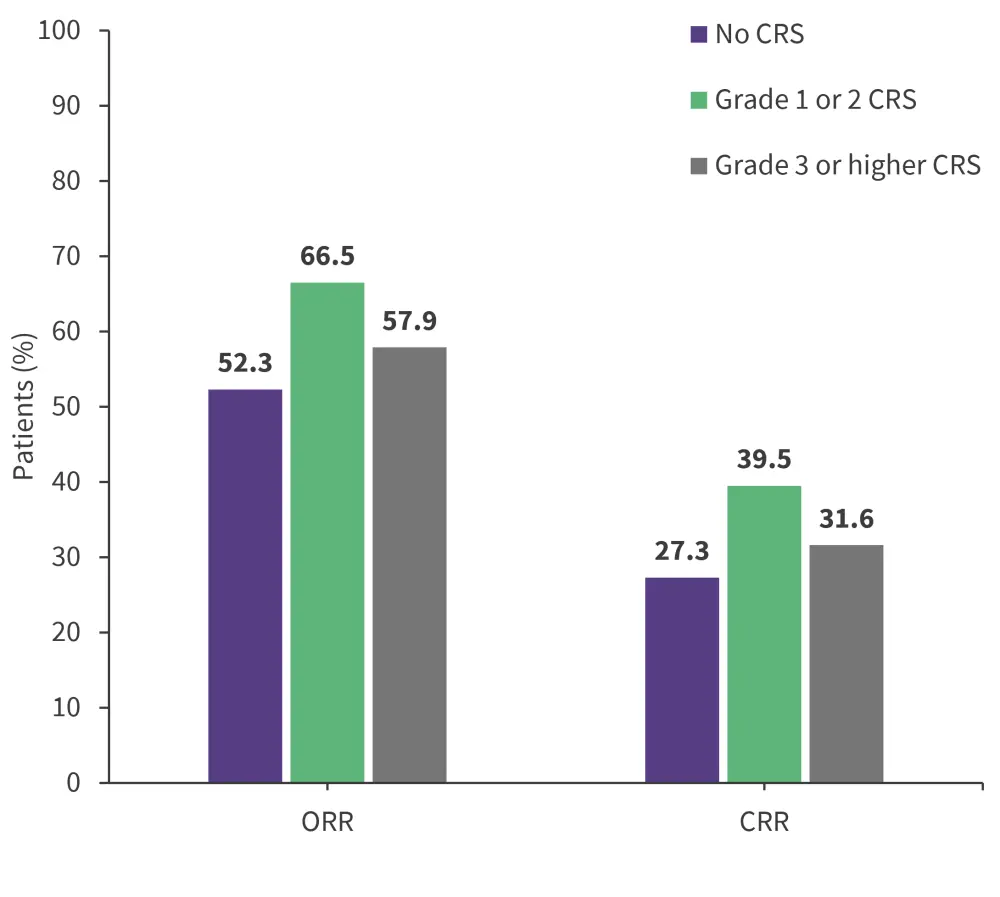
CRR, complete response rate; CRS, cytokine release syndrome; ORR, overall response rate.
*Data from Bhaskar, et al.1
Conclusion
This retrospective analysis showed that patients with aggressive LBCL treated with CD19-directed CAR T cells who develop CRS yield similar objective response, complete response, OS, and PFS outcomes to those who do not develop CRS. Similar to previous findings, the development of CRS was not shown to impact CAR T-cell efficacy; therefore, it is imperative not to discount CAR T-cells early in those who do not develop CRS, and the decision to initiate additional lymphoma-directed therapy post-CAR T-cell therapy should not be based on the development of CRS.
Although the development of CRS did not influence CAR T-cell outcomes, this study highlighted other factors that can impact survival outcomes, such as peak ferritin level of >5000 within 28 days of CAR T-cell infusion, lactate dehydrogenase greater than the upper limit of normal, presence of bulky disease, and receipt of bridging therapy.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


