All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Impact of bridging therapy on CAR T-cell outcomes in patients with LBCL
Do you know... Which of the following factor is positively associated with response to bridging therapy in patients with LBCL?
Intention to treat with chimeric antigen receptor (CAR) T-cell therapy relies on disease management during the manufacturing period of CAR T-cells. Bridging therapy (BT) is administered during this time to control the disease and ensure CAR T-cell infusion can be achieved. The Lymphoma Hub previously reported on the impact of BT on patient outcomes; below, we provide an update on the clinical data guidance.
Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) are approved CAR T-cell therapies for the treatment of patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Previously, the Lymphoma Hub covered key considerations for preparing patients with B-cell non-Hodgkin lymphoma and acute lymphoblastic leukemia for CAR T-cell therapy. Here, we report on considerations for patients with LBCL.
Study design
This retrospective analysis reported data collected from adult patients with R/R LBCL from the United Kingdom National CAR Clinical Panel who underwent leukapheresis for axi-cel or tisa-cel. BT was defined as lymphoma-directed therapy administered between leukapheresis and lymphodepletion (Figure 1). Objectives were complete response and proceeding to CAR T-cell infusion, and comparison of safety and efficacy outcomes (overall response rates, progression-free survival, and overall survival) between patient groups treated with axi-cel and tisa-cel.
Results
Overall, 375 patients with R/R LBCL underwent leukapheresis for CD19 CAR T-cell therapy, and 87% (326/375) received BT (Figure 1).
Figure 1. Patients disposition*
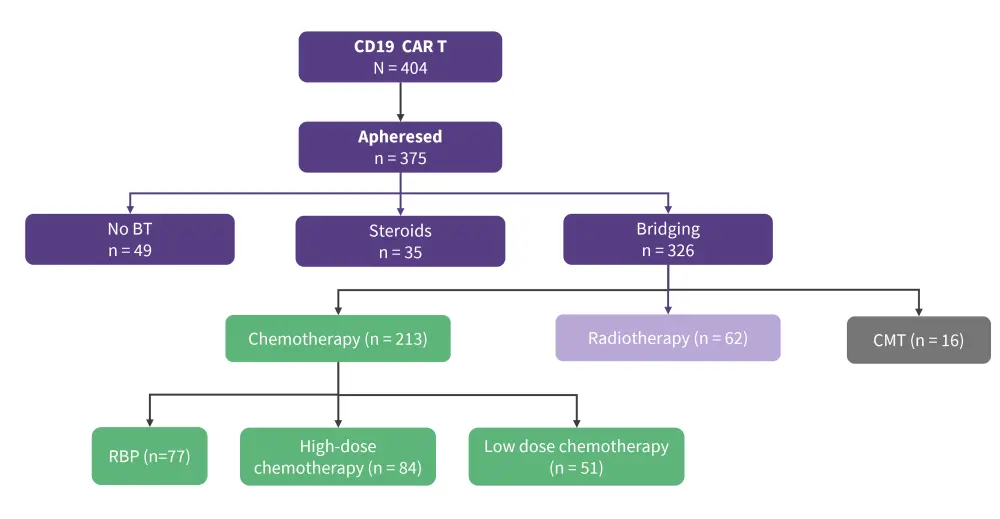
BT, bridging therapy; CAR T, chimeric antigen receptor T; CMT, combined modality therapy; RBP, rituximab-bendamustine-polatuzumab.
*Adapted by Roddie, et al.1
Baseline characteristics per bridging therapy group are summarized in Table 1.
Table 1. Baseline characteristics*
|
ECOG, Eastern Cooperative Oncology Group; LBCL, Large B-cell lymphoma; PMBL, primary mediastinal large B-cell lymphoma; tFL, transformed follicular lymphoma. |
|
||||
|
Characteristic, % (unless otherwise stated) |
No bridging (n = 49) |
Chemotherapy (n = 213) |
Radiotherapy (n = 62) |
Steroids (n = 35) |
Combined modality therapy (n = 16) |
|---|---|---|---|---|---|
|
Age, years |
63 |
60 |
57 |
58 |
63 |
|
Male |
61.2 |
60.0 |
59.7 |
62.9 |
50 |
|
Female |
38.8 |
37.1 |
40.3 |
40 |
50 |
|
Disease type |
|||||
|
De novo LBCL |
63.3 |
69.5 |
64.5 |
68.6 |
56.3 |
|
PMBL |
6.1 |
5.2 |
6.5 |
0 |
12.5 |
|
tFL |
24.5 |
18.3 |
25.8 |
28.6 |
25.0 |
|
t-Other |
6.1 |
7.0 |
3.2 |
2.9 |
6.3 |
|
ECOG Performance Status |
|||||
|
1 |
63.3 |
37.6 |
51.6 |
51.4 |
18.8 |
|
2 |
36.7 |
62.4 |
48.4 |
48.6 |
81.3 |
|
Stage |
|||||
|
Stage 1–2 |
30.4 |
15.6 |
29.5 |
25.7 |
13.3 |
|
Stage 3–4 |
69.6 |
84.4 |
70.5 |
74.3 |
86.7 |
Response to BT and impact on CAR T-cell infusion
- Overall, complete response to BT was achieved by 23 patients (four after radiotherapy and 19 after chemotherapy).
- Patients who underwent high-dose chemotherapy were less likely to reach CAR T-cell infusion due to disease progression, central nervous system relapse, or death.
- Overall response rates were higher in patients treated with radiotherapy and rituximab-bendamustine-polatuzumab chemotherapy than low-dose chemotherapy, high-dose chemotherapy, and combined modality therapy (Figure 2).
Figure 2. Overall response rates in patients according to BT*
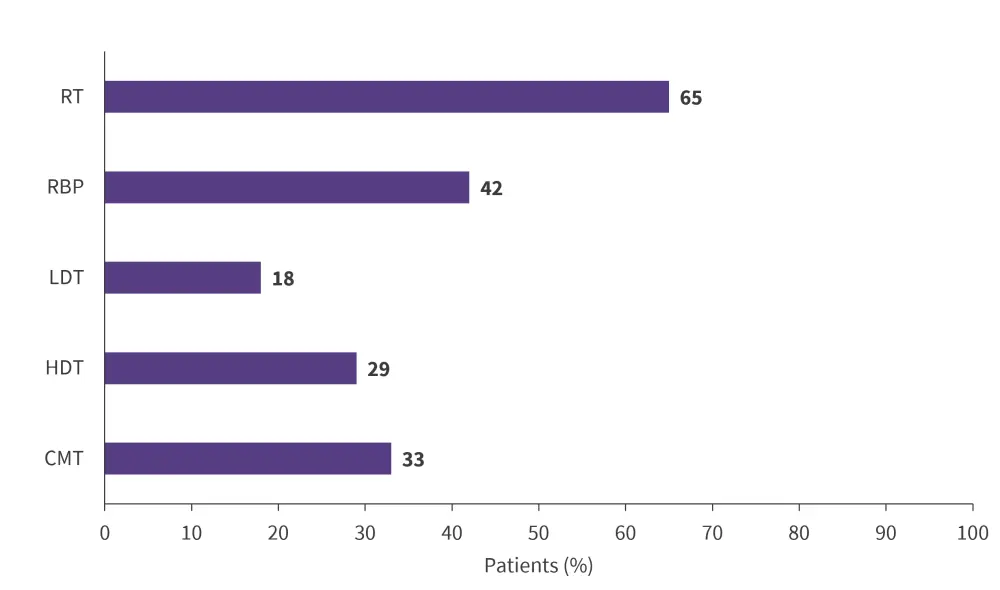
BT, bridging therapy; CMT, combined modality therapy; HDT, high-dose chemotherapy; LDT, low-dose chemotherapy; RBP, rituximab-bendamustine-polatuzumab; RT, radiotherapy.
Adapted from Roddie, et al.
Axi-cel vs tisa-cel outcomes
- Of the 23 patients who achieved complete response, 13 received axi-cel therapy and eight received tisa-cel therapy.
- Objective response rate of CAR T-cell therapy was highest in patients bridged with radiotherapy (Figure 3).
- Comparing BT responders vs non-responders, progression-free survival following tisa-cel was significantly higher (p < 0.001) than axi-cel (p = 0.071).
Figure 3. Objective response rate to CAR T-cell therapy with or without BT*
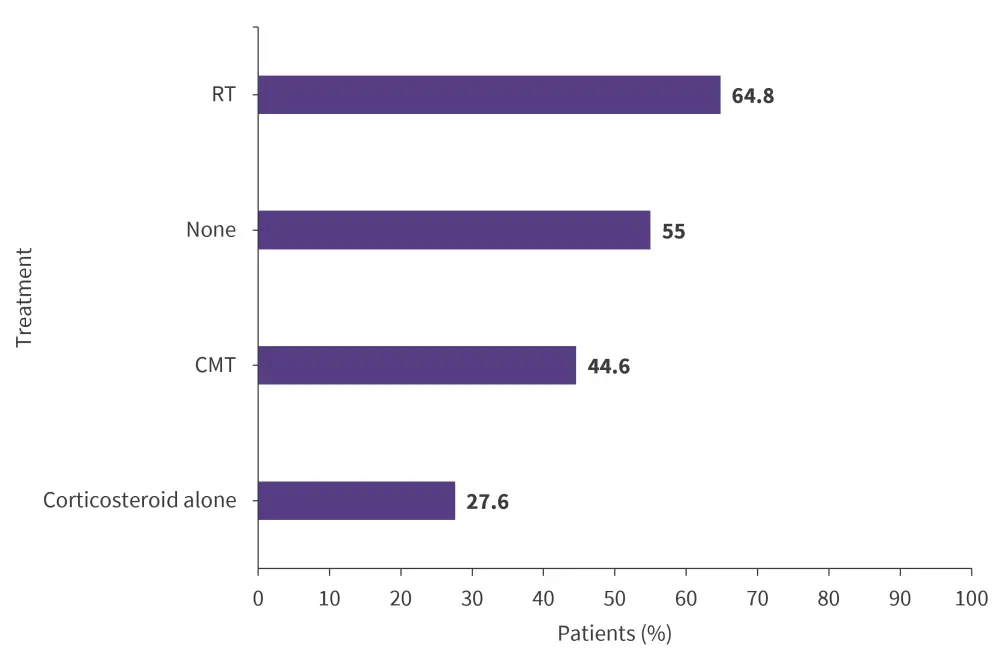
CAR T, chimeric antigen receptor T-cell; CMT, combined modality therapy; RT, radiotherapy.
Adapted from Roddie, et al.
Factors associated with response to BT
The multivariate analysis demonstrated that factors independently associated with a higher probability of response to bridging therapy were:
- response to the last line of therapy (overall response, 2.17; 95% confidence interval [CI], 1.11–4.22; p = 0.023);
- absence of bulky disease (>7.5cm diameter; OR, 0.49; 95% CI, 0.25–0.98; p = 0.045); and
- use of rituximab-bendamustine-polatuzumab (overall response compared to LDT/HDT, 2.21; 95% CI, 1.21–4.05; p = 0.010).
Safety
- No difference in cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, use of corticosteroids or intensive care unit admission observed across BT cohorts.
- Rates of Grade ≥3 thrombocytopenia (one month after CAR T-cell therapy) were higher in patients who underwent bridged chemotherapy compared with other BTs and no bridged therapy (56% vs 10–31.9% and 20.6%).
- Rates of Grade ≥3 neutropenia were higher in BT groups (one month after treatment) compared with patients who had not been treated with BT (40–45% vs 27.3%).
Conclusion
This study demonstrates the use of BT as a safe and effective strategy to improve CAR T-cell infusion, regardless of the type of BT used. No new safety signals were reported. This study suggests that BT in LBCL should be carefully planned toward optimal response and disease debulking, to improve patient outcomes. Eligible patients should consider receiving a regimen containing polatuzumab and where possible, additional lines of BT should be considered if BT fails to produce a complete or partial response before tisa-cel administration.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



