All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Updated results from the ZUMA-5 trial of axi-cel for patients with relapsed/refractory follicular lymphoma
The treatment of follicular lymphoma (FL) is heterogenous in clinical practice and there is a lack of uniform treatment options. Chimeric antigen receptor (CAR) T-cells have shown promise for the treatment of FL, and thus were investigated in the ZUMA-5 trial (NCT03105336). This is a prospective trial designed to test an anti-CD19 CAR T-cell therapy, axicabtagene ciloleucel (axi-cel), in patients with relapsed/refractory (R/R) indolent non-Hodgkin lymphoma who have failed two or more prior lines of therapy. During the 26th Congress of the European Hematology Association (EHA2021), the updated results of the ZUMA-5 trial were presented by John Gribben.1
The interim analysis of the trial has been previously reported by the Lymphoma Hub and can be found here; the study design can be found here.
Study design
This study used cross-study comparisons of the retrospective SCHOLAR-5 study with a prospective clinical trial, the phase II ZUMA-5 trial. The design of the study is shown in Figure 1. SCHOLAR-5 is an external control cohort that was produced to allow for comparisons with the patients on the ZUMA-5 trial. To make the characteristics between the cohorts balanced, propensity score weights were used, after which the only category that was significantly different between groups was the Eastern Cooperative Oncology Group (ECOG) score (p < 0.05). As all patients were either 0 or 1, the investigators did not deem this to be clinically significant.
Figure 1. Study design*
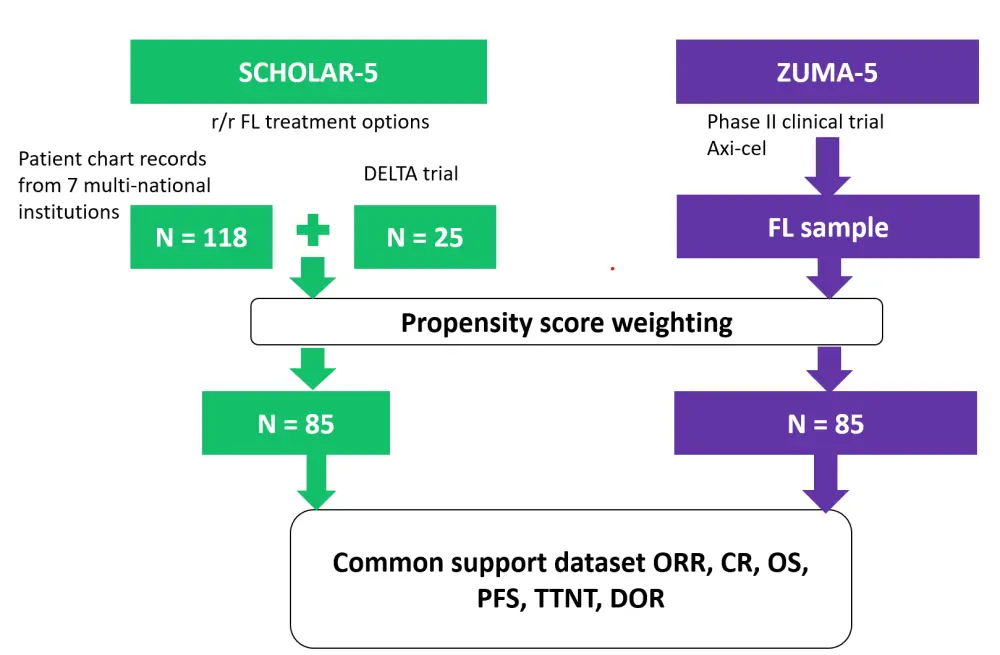
CR, complete response; DOR, duration of response; FL, follicular lymphoma; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTNT, time to next treatment.
*Adapted from Ghione et al.1
In the ZUMA-5 cohort, 73.3% of patients had been refractory to the previous line of therapy and 24.4% had undergone a stem cell transplant. Patients in this cohort received a mean of 3.6 previous lines of therapy (Table 1).
Table 1. Baseline patient characteristics following propensity score weighting*
|
Characteristic |
ZUMA-5 |
SCHOLAR-5 after weighting |
|
Median age, years (range) |
62 (34−79) |
61 (36−89) |
|
Male, % |
55.8 |
61.9 |
|
POD24, % |
57.0 |
55.9 |
|
Prior lines of therapy, mean (SD) |
3.6 (1.6) |
3.5 (1.6) |
|
Refractory to prior line, % |
73.3 |
76.6 |
|
Prior SCT, % |
24.4 |
28 |
|
Size of the largest node (cm), mean (SD) |
5.2 (2.9) |
4.9 (2.7) |
|
Time since last therapy (months), mean (SD) |
8.4 (11.7) |
7.7 (13.3) |
|
Time since diagnosis (months), mean (SD) |
76.1 (62.8) |
82.2 (58.5) |
|
ECOG, % |
||
|
0 |
59.3 |
29 |
|
1 |
40.7 |
71 |
|
ECOG, Eastern Cooperative Oncology Group; POD24, progressed within 24 months of initiating first-line chemoimmunotherapy; SCT, stem cell transplant; SD, standard deviation. |
||
Results
The overall response rate (ORR) was significantly higher in the ZUMA-5 group compared with the SCHOLAR-5 group (Table 2). With a median follow-up of 26.2 months, the median progression-free survival (PFS) in the SCHOLAR-5 group was 12.7 months. The median follow-up in the ZUMA-5 cohort was 23.3 months, while the median PFS was not reached (23.5, NR), which was significantly improved in comparison with the SCHOLAR-5 group (p < 0.0001).
Table 2. Survival outcomes in the ZUMA-5 and SCHOLAR-5 cohorts in patients who failed ≥2 prior lines of therapy*
|
CR, complete response; HR, hazard ratio; NE, not estimable; NR, not reached; OR, odds ratio; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTNT, time to next treatment. |
|||||
|
Outcome |
ZUMA-5 |
SCHOLAR-5 |
OR (95% CI) |
HR (95% CI) |
p value |
|---|---|---|---|---|---|
|
ORR, % |
94.2 |
49.9 |
16.2 |
— |
< 0.0001 |
|
CR, % |
79.1 |
29.9 |
8.9 |
— |
< 0.0001 |
|
Median PFS, months (range) |
NR |
12.7 |
— |
0.30 |
< 0.001 |
|
Median OS, months (range) |
NR |
59.8 |
— |
0.42 |
0.0125 |
|
Median TTNT, months (range) |
NR |
14.4 |
— |
0.42 |
< 0.001 |
A subset of patients who had received ≥3 previous lines of therapy were also examined, and axi-cel proved to be beneficial in this group as well. In the ZUMA-5 cohort (n = 60) and in the SCHOLAR-5 cohort (n = 59), the odds ratio (OR) was 28.13 for the ORR. With respect to complete response (CR), the OR was 15.42.
Conclusion
Axi-cel demonstrated a significantly improved ORR, CR, PFS, and time to next treatment in the ZUMA-5 cohort compared with the SCHOLAR-5 group. In addition, the hazard ratio for overall survival was 0.42 (p = 0.01), indicating a decreased risk of an event, suggesting that axi-cel is suitable for treating patients with R/R FL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

