All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
CAR T-cell updates from ZUMA-5 and ELARA trials
Relapse following standard therapeutic approaches is common in patients with advanced-stage, indolent non-Hodgkin lymphomas (iNHL). Two autologous anti-CD19 chimeric antigen receptor (CAR) T-cell products, axicabtagene ciloleucel (axi-cel), and tisagenlecleucel (tisa-cel), both approved for the treatment of relapsed/refractory (R/R) large B-cell lymphoma (LBCL), are currently under investigation in the phase II ZUMA-5 (NCT03105336) and ELARA (NCT03568461) trials for patients with iNHL, including follicular lymphoma (FL), and marginal zone lymphoma (MZL).
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, updates on both ZUMA-5 and ELARA trials were presented by Caron Jacobson,1 and by the Lymphoma Hub Executive Steering Committee member Nathan Fowler,2 respectively. Here, we combine key points from these sessions.
ZUMA-5 trial1
ZUMA-5 is a multicenter, single arm, phase II study of axi-cel for the treatment of R/R FL and MZL after ≥ 2 prior lines of therapy. An interim analysis of 96 patients in the ZUMA-5 trial was previously covered on the Lymphoma Hub. In the current analysis, 151 patients with R/R iNHL were enrolled, and 124 patients with FL, and 22 patients with MZL were treated (total = 146).
Selected patient characteristics at baseline for the expanded cohort were as follows:
- Median age was of 61 years for all patients (range, 34–79)
- Patients ≥ 65 years of age: 35%
- Most patients had Stage III–IV disease (86%)
- The population was heavily pretreated
- Median number of prior therapies: 3 (1–10)
- ≥ 3 prior therapies: 64%
Other patient characteristics were similar to those reported in the previous analysis.
Results
Efficacy
The efficacy analysis included 104 evaluable patients (FL, n = 84; MZL, n = 20) with a median follow-up of 17.5 months (range, 1.4–31.6 months).
The overall response rate (ORR) for all patients was 92% (95% CI, 85–97), and complete response (CR) rate was 76% (95% CI, 67–84).
- Median time to first response was 1 month (range, 0.8–3.1)
- ORR was 94% and 85% for patients with FL and MZL, respectively (Figure 1)
- ORR was consistent among different subgroups
- Of the 25 patients with FL who initially had a partial response, 52% converted to CR after a median of 2.2 months (range, 1.9–11.2)
Figure 1. Best overall response rates1
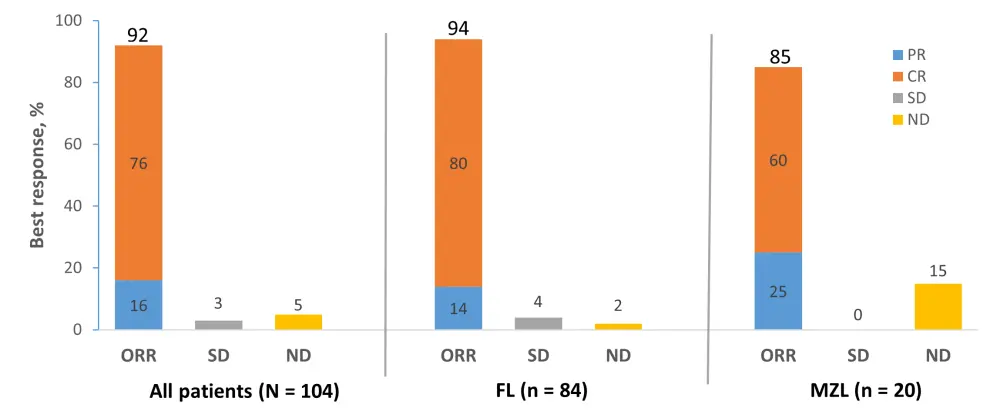
CR, complete response; FL, follicular lymphoma; MZL, marginal zone lymphoma; ND, undefined/not done; ORR, objective response rate; PR, partial response; SD, stable disease.
- With a median follow-up of 17.5 months, estimated median duration of response (DOR) for all patients was not reached (Table 1)
- At data cutoff, 64% of patients with FL maintained their response
-
- Patients with CR were more likely to maintain their response than patients with PR (78% vs 17%, respectively)
- Median progression-free survival (PFS) and overall survival (OS) were not reached for all patients (Table 1)
Table 1. Key secondary efficacy results1
|
CI, confidence interval; DOR, duration of response; FL, follicular lymphoma; MZL, marginal zone lymphoma; NE, not estimable; OS, overall survival; PFS, progression-free survival. |
|||
|
Outcome |
FL |
MZL |
All patients |
|---|---|---|---|
|
Median follow-up, months (range) |
18.5 (12.2–31.6) |
12.1 (1.4–26.8) |
17.5 (1.4–31.6) |
|
Median DOR, months (95% CI) |
NE (20.8–NE) |
10.6 (8.1–NE) |
NE (20.8–NE) |
|
12-month DOR rate, % (95% CI) |
77.0 (65.5–85.1) |
NE (NE–NE) |
71.7 (60.7–80.1) |
|
Median PFS, months (95% CI) |
NE (23.5–NE) |
11.8 (9.1–NE) |
NE (23.5–NE) |
|
12-month PFS, % (95% CI) |
77.5 (66.6–85.2) |
45.1 (15.2–71.4) |
73.7 (63.3–81.6) |
|
Median OS, months (95% CI) |
NE (NE–NE) |
NE (NE–NE) |
NE (NE–NE) |
|
12-month OS, % (95% CI) |
92.8 (84.7–96.7) |
92.9 (59.1–99.0) |
92.9 (85.6–96.5) |
Safety
The safety analysis included all treated patients (n = 146), with a median follow-up of 15.1 months (range, 0.5–31.6 months). Table 2 summarizes safety outcomes of interest.
Table 2. Safety outcomes1
|
AE, adverse events, CRS, cytokine release syndrome; FL, follicular lymphoma; MZL, marginal zone lymphoma. |
|||
|
Outcome |
FL |
MZL |
All patients |
|---|---|---|---|
|
Any AE, % |
99 |
100 |
99 |
|
Grade ≥ 3 CRS*, % Median time to onset, days (range) |
6 4 (1–15) |
9 4 (1–9) |
7 4 (1–15) |
|
Grade ≥ 3 neurologic events†, % Median time to onset, days (range) |
15 7 (1–177) |
41 7 (3–19) |
19 7 (1–177) |
- The rate of Grade ≥ 3 adverse events (AEs) was 86% (n = 126), the most common being cytopenia (70%), and infections (16%)
- Of note, the rate of Grade ≥ 3 neurologic events was lower in the FL cohort (15%) compared with the MZL cohort (41%)
- Grade 5 AEs occurred in three patients. One patent died of multisystem organ failure (Day 7) due to cytokine release syndrome (CRS). This event was considered related to axi-cel therapy
- No patients had ongoing CRS; however, Grade 1 and 2 neurological events were still ongoing in six patients at the data cutoff date
The results of pharmacokinetic and pharmacodynamic analyses were similar to those reported in the previous analysis.
Conclusion
Axi-cel is a promising approach for the treatment of patients with R/R iNHL, demonstrating high rates of durable responses that were consistent among patients with high-risk features. The safety profile was manageable and appeared to be favorable for FL compared with data previously reported for LBCL. Based upon the manageable safety profile, the possibility of outpatient treatment is to be investigated.
ELARA trial2
ELARA is a single-arm, multicenter, phase II study evaluating the efficacy and safety of tisa-cel in patients with R/R FL. The trial recently met its primary endpoint (CR rate); secondary endpoints include overall response rate (ORR), DOR, PFS, OS, and safety.
Study design
Eligibility criteria:
- ≥ 18 years of age
- Grade 1, 2, or 3A FL
- R/R disease
- No histological transformation/FL3B
- Anti-CD19 or allogeneic HSCT-naïve
The study design is depicted in Figure 2. First efficacy assessment was performed at Month 3. Efficacy and safety follow-up will be done every 3 months for the first 12 months, and then every 6 months until study completion.
Figure 2. Study design2
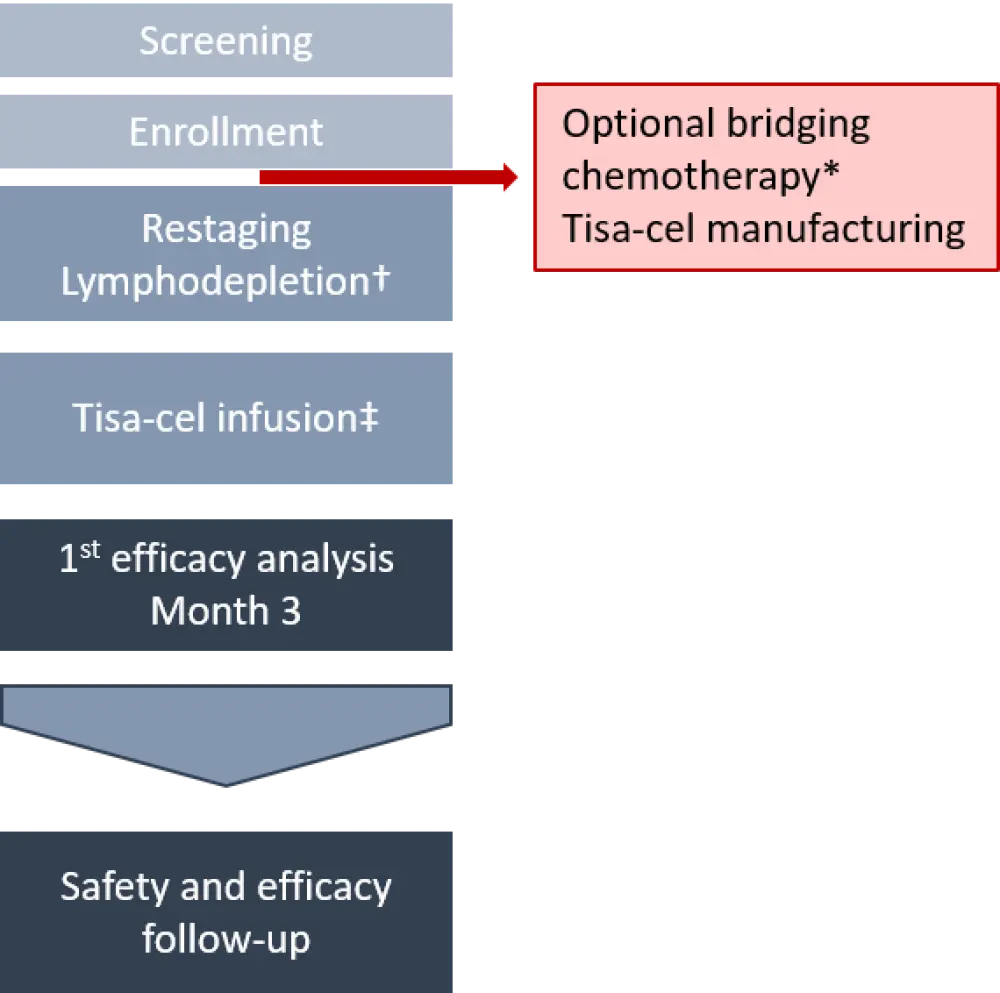
O-Benda, obinutuzumab and bendamustine; R2, lenalidomide and rituximab; R-CHOP; rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-GemOx, rituximab, gemcitabine, oxaliplatin; tisa-cel, tisagenlecleucel.
*Administered in 43.3% of patients. The most common regimens included R-GemOx, R-CHOP, and O-Benda. A few patients received idelalisib and R2.
†Fludarabine (daily IV dose of 25 mg/m2 for 3 days) plus cyclophosphamide (daily IV dose of 250 mg/m2 for 3 days), OR bendamustine (daily IV dose of 90 mg/m2 for 3 days).
‡Dose range: 0.6–6 × 108 CAR+ viable T cells in a single IV infusion.
Results
A total of 97 patients have been enrolled, with a median age of 57.0 years (range, 29–73 years). The study population was heavily pretreated, with a median of four prior therapies (range, 2–13). Baseline characteristics are presented in Table 3.
Table 3. Baseline characteristics2
|
Auto-HSCT, autologous hematopoietic stem cell transplantation; ECOG PS, European Cooperative Oncology Group performance status; FLIPI, follicular lymphoma international prognostic factor index; mAb, monoclonal antibody; PI3K, phosphoinositide 3-kinase inhibitor; POD24, progression of disease within 24 months. |
|
|
Characteristic, % |
N = 97 |
|---|---|
|
ECOG PS before infusion 0 1 |
56.7 39.2 |
|
Stage III–IV disease |
83.5 |
|
FLIPI ≥ 3 |
59.8 |
|
Prior therapies ≥ 3 Auto-HSCT Anti-CD20 mAb and alkylating agents PI3K inhibitors Lenalidomide and rituximab |
76.3 36.1 100 20.6 17.3 |
|
POD24 from first anti-CD20 mAb-based therapy |
59.8 |
|
Refractory to last line of therapy |
77.3 |
|
Refractory to ≥ 2 regimens |
75.5 |
Efficacy
Median follow-up was 9.9 months (range, 6.0–15.6), and median DOR was not reached. Fifty-two patients were evaluable. Efficacy outcomes are presented in Table 4.
Table 4. Outcomes2
|
CR, complete response; ORR, overall response rate; PFS, progression-free survival; PR, partial response |
|
|
Outcomes, % |
N = 52 |
|---|---|
|
CR |
65.4 |
|
PR |
17.3 |
|
ORR (CR + PR) |
82.7 |
|
6-month PFS (95% CI) |
73.2 (58.2–83.5) |
- Median infused dose of tisa-cel was 2.06 × 108 CAR+ viable T cells
- At the time of data cutoff, 69% of patients maintained their responses
- Amongst patients with PR, 44% converted to CR
- Median PFS and OS were not reached
Safety
Median follow-up for safety was 6.5 months (range, 0.2–15.6). Most patients (94.8%) experienced an AE of any grade, and 73.2% of these were suspected to be related to tisa-cel. The frequency of Grade 3–4 AEs was 70.1%, of which 38.1% were suspected to be related to tisa-cel. Table 5 provides the Grade ≥ 3 AEs of special interest. There were three deaths, and all were due to disease progression.
Table 5. Grade ≥ 3 AEs of special interest2
|
AEs, adverse events. |
|
|
Grade ≥ 3 AEs*, % |
N = 97 |
|---|---|
|
Neutropenia |
24.7 |
|
Anemia |
12.4 |
|
Thrombocytopenia |
8.2 |
|
Infections |
4.1 |
|
Serious neurological adverse reactions |
1.0 |
Median onset of neurological events was 8.5 days (range, 4–190), and only one Grade ≥ 4 immune effector cell-associated neurotoxicity syndrome (ICANS) was reported. Median onset of CRS was 4.0 days (1–14), and there were no Grade ≥ 3 CRS events. All neurological events and CRS were managed with suitable approaches including tocilizumab and corticosteroids.
Conclusion
This analysis demonstrated that tisa-cel may be an effective option for heavily pretreated patients with R/R FL. CR and ORR rates were 65% and 83%, respectively. Median DOR, PFS, and OS were not reached for all patients. Safety analysis did not produce any new safety concerns. There were no deaths considered related to the study treatment.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

