All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Interim analysis of the phase II ZUMA-5 study of axicabtagene ciloleucel in patients with R/R iNHL
During an oral abstract session at the 2020 American Society of Clinical Oncology (ASCO) Annual Meeting, Caron Jacobson from the Dana-Farber Cancer Institute, Boston, US, presented abstract 8008 on the ZUMA-5 study (NCT03105336). This was a phase II study of axicabtagene ciloleucel (axi-cel) in patients with relapsed/refractory indolent non-Hodgkin lymphoma (R/R iNHL).1
Advanced stage iNHL, including follicular lymphoma (FL) and marginal zone lymphoma (MZL) are largely incurable with conventional therapies. Many patients experience multiple relapses over the natural history of their disease, as well as a shortened remission duration with subsequent therapies. Axi-cel is an anti-CD19, autologous, chimeric antigen receptor (CAR) T-cell therapy, with a CD28 costimulatory domain. It has been approved in the US and in the EU for the treatment of R/R aggressive large B-cell NHL after ≥ 2 prior lines of systemic therapy. Approval was based on the results of the ZUMA-1 study; the long-term results can be found on the Lymphoma Hub here.
Study design and patient characteristics1,2
- Multi-center, single arm, phase II study of axi-cel for the treatment of R/R FL and MZL after ≥ 2 prior lines of therapy
- Adults (≥ 18 years) with R/R FL (Grades 1–3a), or MZL (nodal or extranodal) and an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0–1 were included in the study
- Prior lines of therapy included at least one anti-CD20 monoclonal antibody combined with an alkylating agent
- Patients underwent leukapheresis (N = 148) and received conditioning chemotherapy (n = 140) with fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2 for 3 days, starting 5 days prior to CAR T-cell infusion
- Axi-cel was given as an infusion at 2 × 106 CAR T-cells/kg
- Primary endpoint: Objective response rate (ORR) by central review
- Secondary endpoints: Complete response (CR), duration of response, progression-free survival, overall survival (OS), safety, and blood levels of cytokines and CAR T-cells
- As of December 16, 2019, 140 patients (n = 124, FL; n = 16, MZL) who received axi-cel were evaluable for safety analysis and had a median follow-up time of 15.3 months (range, 1.9–28.8)
- 96 patients (n = 80, FL with ≥ 9 months of follow-up; n = 16, MZL with ≥ 1 month of follow-up) were evaluable for efficacy and had a median follow-up time of 12.8 months (range, 1.9–28.8)
- Baseline patient characteristics can be seen in Table 1
Table 1. Baseline patient characteristics1
|
ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; FLIPI, follicular lymphoma international prognostic factor index; GELF, Groupe d’Etude des Lymphomes Folliculaires; iNHL, indolent non-Hodgkin lymphoma; MZL, marginal zone lymphoma; PI3Ki, phosphoinositide 3-kinase inhibitor; POD24, progression of disease < 24 months; SCT, stem cell transplant *patients with iNHL who progressed ≤ 6 months of completion of the most recent prior therapy |
|||
|
Characteristic |
All patients N = 96 |
FL n = 80 |
MZL n = 16 |
|---|---|---|---|
|
Age, years |
|
|
|
|
Median (range) |
63 (34–79) |
62 (34–79) |
67 (52–77) |
|
≥ 65, % |
42 |
36 |
69 |
|
Male/female, % |
49/41 |
54/46 |
24/76 |
|
ECOG Performance Status 1, % |
41 |
41 |
38 |
|
Stage IV disease, % |
52 |
46 |
81 |
|
FLIPI ≥ 3, % |
51 |
48 |
69 |
|
High tumor bulk (GELF criteria), % |
49 |
50 |
44 |
|
Prior therapies, % |
|
|
|
|
Median (range) |
3 (2–9) |
3 (2–9) |
3 (2–8) |
|
≥ 3, % |
70 |
70 |
69 |
|
PI3Ki therapy, % |
33 |
33 |
38 |
|
Autologous SCT, % |
23 |
24 |
19 |
|
Refractory disease*, % |
73 |
74 |
69 |
|
POD24 from first anti-CD20 therapy, % |
54 |
56 |
44 |
Results1
Efficacy
- The overall ORR (Figure 1) for all patients was 93% (95% CI, 86–97) with a CR rate of 80% (95% CI, 77–88)
- Median time to first response was 1 month (range, 0.8–3.1)
- ORR was 95% vs 81% for patients with FL and MZL, respectively
Of the patients with FL (n = 80), 10 patients (13%) had an initial response of PR at Week 4 and then later converted to CR
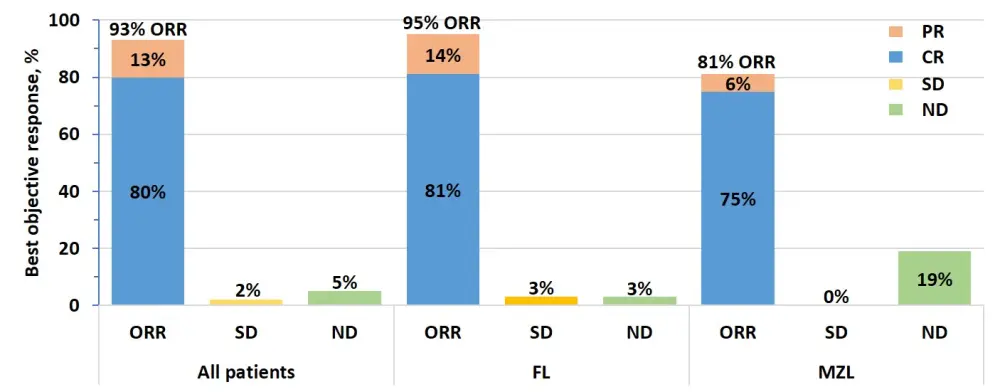
Figure 1. Objective response rates. CR, complete response; FL, follicular lymphoma; MZL, marginal zone lymphoma; ND, undefined/not done; ORR, objective response rate; PR, partial response; SD, stable disease
- The median follow-up, estimated median duration of response, progression-free survival, and 12-month OS rates can be seen in Table 2
- Amongst the responding patients in the FL cohort, nearly 80% maintained their response past 12 months
- In the patients with FL, 80% of patients in CR and 18% in PR had ongoing responses
- In the MZL cohort very few patients reached follow-up past 6 to 9 months
- The median OS was not reached
Table 2. Key secondary efficacy results1
|
CI, confidence interval; DOR, duration of response; FL, follicular lymphoma; MZL, marginal zone lymphoma, NE, not estimable; OS, overall survival; PFS, progression-free survival |
|||
|
Result |
All patients N = 96 |
FL n = 80 |
MZL n = 16 |
|---|---|---|---|
|
Median follow-up, months (range) |
15.3 |
16.0 (10.1–28.8) |
11.1 (1.9–23.9) |
|
Estimated median DOR months (95% CI) |
20.8 |
20.8 (19.7–NE) |
10.6 (4.6–11.1) |
|
Median PFS, months (95% CI) |
23.5 (22.8–NE) |
23.5 (22.8–NE) |
11.8 (6.0–12.0) |
|
12-month OS rate, % (95% CI) |
94.3 (86.8–97.6) |
93.4 (84.9–97.2) |
100 (NE–NE) |
Safety
- 140 patients (n = 124, FL; n = 16, MZL) were evaluable for safety and 99% of patients experienced ≥ 1 adverse event (AE) (Table 3)
Table 3. TEAEs that occurred in ≥ 25% of patients1
|
FL, follicular lymphoma; MZL, marginal zone lymphoma; TEAE, treatment emergent adverse event |
|||
|
TEAE |
All patients N = 140 |
FL n = 124 |
MZL n = 16 |
|---|---|---|---|
|
Any, % |
99 |
99 |
100 |
|
Pyrexia |
84 |
83 |
94 |
|
Hypotension |
49 |
48 |
56 |
|
Fatigue |
44 |
41 |
69 |
|
Headache |
44 |
43 |
50 |
|
Nausea |
39 |
35 |
69 |
|
Neutropenia |
36 |
39 |
19 |
|
Anemia |
35 |
35 |
31 |
|
Sinus tachycardia |
34 |
33 |
38 |
|
Decreased neutrophil count |
29 |
25 |
63 |
|
Tremor |
29 |
27 |
44 |
|
Constipation |
29 |
28 |
31 |
|
Chills |
28 |
25 |
50 |
|
Diarrhea |
28 |
26 |
44 |
|
Decreased appetite |
25 |
23 |
44 |
- 85% of patients experienced ≥ Grade 3 AEs, with neutropenia being the most common (34%) followed by decreased neutrophil count (28%) and anemia (22%)
- In patients with FL, two developed Grade 5 AEs: Multisystem organ failure in the context of cytokine release syndrome (CRS) that was deemed related to treatment, and aortic dissection that was deemed unrelated to treatment
- 79% of patients experienced CRS of any grade (Table 4)
- One patient with MZL experienced Grade 4 CRS and one patient with FL experienced Grade 5 CRS
- At the data cutoff, no patients had ongoing CRS
Table 4. CRS*1
|
AE, adverse event; CRS, cytokine release syndrome; FL, follicular lymphoma; MZL, marginal zone lymphoma *CRS was graded by the Lee criteria |
|||
|
Parameter |
All patients N = 140 |
FL n = 124 |
MZL n = 16 |
|---|---|---|---|
|
Any Grade, % |
79 |
77 |
100 |
|
≥ Grade 3, % |
8 |
7 |
13 |
|
Most common symptom of any grade, % |
|
|
|
|
Pyrexia |
96 |
97 |
94 |
|
Hypotension |
41 |
41 |
38 |
|
AE management, % |
|
|
|
|
Tocilizumab |
47 |
44 |
69 |
|
Corticosteroids |
17 |
16 |
25 |
|
Median time to onset, days (range) |
4 (1–15) |
4 (1–15) |
4 (1–9) |
|
Median duration of events, days (range) |
6 (1–27) |
6 (1–27) |
6 (2–14) |
|
Patients with resolved events, % |
99 |
99 |
100 |
- 58% of patients experienced neurological events of any grade (Table 5)
- There were no Grade 5 neurological events
- Grade 4 neurological events were experienced by two patients with FL
- Four patients had ongoing neurological events at data cuttoff of Grade 1 and 2 paraesthesia, Grade 1 attention disturbance and Grade 1 memory impairment
Table 5. Neurotoxicity1
|
AE, adverse event; CRS, cytokine release syndrome; FL, follicular lymphoma; MZL, marginal zone lymphoma |
|||
|
Parameter |
All patients N = 140 |
FL n = 124 |
MZL n = 16 |
|---|---|---|---|
|
Any Grade, % |
58 |
55 |
81 |
|
≥ Grade 3, % |
17 |
15 |
38 |
|
Most common symptom of any grade, % |
|
|
|
|
Tremor |
51 |
50 |
54 |
|
Confusion |
41 |
41 |
38 |
|
AE management, % |
|
|
|
|
Tocilizumab |
7 |
6 |
13 |
|
Corticosteroids |
34 |
29 |
69 |
|
Median time to onset, days (range) |
7 (1–177) |
7 (1–177) |
7 (3–19) |
|
Median duration of events, days (range) |
14 (1–452) |
14 (1–452) |
13 (2–81) |
|
Patients with resolved events, % |
95 |
96 |
92 |
Blood levels of cytokines and CAR T-cells
- Median time to peak of anti-CD19 CAR T-cell levels was 8 days (range, 8–371) post-infusion
- Peak CAR T-cell levels were slightly higher, and occurred later (by approximately a week), in the MZL cohort
- Anti-CD19 CAR T-cells were detectable in 87% of patients with evaluable samples (n = 15) at 18 months of follow-up
- Peak CAR T-cell levels were similar amongst responders in patients with FL and MZL, however, there were insufficient numbers of nonresponding patients (n = 2, FL; n = 0, MZL) to adequately assess a relationship between peak CAR T-cell levels and objective response
- Patients with FL who had an ongoing response at 9 months had greater CAR T-cell expansion compared with patients with relapsing or primary refractory disease
- In the FL cohort, peak CAR T-cell expansion was associated with ≥ Grade 3 CRS (p = 0.0088) and neurologic events (p = 0.0076)
- Serum cytokines were measured over time and correlated with ≥ Grade 3 CRS and neurotoxicity
- Patients with FL who experienced ≥ Grade 3 CRS and/or neurotoxicity had higher peak levels of several cytokines including interferon-γ, interleukin 2 (IL-2), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and monocyte chemotactic protein-1 (MCP-1)
Conclusions
- Axi-cel demonstrated high rates of durable responses
- The lower response rate in the MZL may have been attributed to the fact that 19% of patients were found to have no measurable disease by central radiology review prior to CAR T-cell infusion
- The safety profile was manageable, generally reversible, and consistent with the ZUMA-1 study
- The incidence of any grade CRS and high-grade CRS appeared to be higher for the MZL cohort
- The median time to onset of CRS (4 days), was later than the one seen with axi-cel in the ZUMA-1 study (2 days)
- The incidence of any grade or high-grade neurotoxicity was also higher in the MZL cohort (Grade 3/4 neurotoxicity of 15% vs 38%, FL and MZL cohorts, respectively)
Axi-cel appears to be a promising therapeutic approach for patients with R/R iNHL, however a longer follow-up is needed to determine the durability of response
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

