All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Updated results from ZUMA-2 trial investigating KTE-X19 in R/R mantle cell lymphoma
During the 47th Annual Meeting of the European Society of Blood and Marrow Transplantation (EBMT), investigators presented results from the latest analyses of the pivotal phase II ZUMA-2 trial assessing KTE-X19, an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in patients with mantle cell lymphoma (MCL).
Disease progression following treatment with Bruton’s tyrosine kinase inhibitors (BTKi) is associated with poor prognosis, with a short overall survival (OS) of approximately 6 months.1 Different morphological characteristics, such as blastoid or pleomorphic MCL, are also associated with inferior clinical outcomes.1 The ZUMA-2 trial assessed KTE-X19 in patients with MCL who had relapsed or refractory (R/R) disease following 1–5 prior lines of therapy, including a BTKi.
The first part of this article summarizes data comparing the pharmacological profile and efficacy of KTE-X19 in subgroups by MCL morphology and previous exposure to BTKi.1 Later on, we summarize ≥1-year follow-up data on the long-term efficacy, safety, and pharmacology of KTE-X19 in the study population.2 Both sessions were presented by Michael Wang at the EBMT meeting.
Pharmacology and efficacy by disease morphology and prior BTKi exposure
Subgroups were defined as follows:
- Morphological characteristics: classical (n = 40), blastoid (n = 17), and pleomorphic MCL (n = 4)
-
- Patient characteristics were generally similar among these subgroups.
- Patients with pleomorphic MCL had higher median tumor load than patients with classical and blastoid MCL.
- Ki-67 proliferation index ≥50% was observed in about half of patients with classical MCL (56%) and all patients in other two subgroups (100%).
- Prior BTKi exposure: ibrutinib only (n = 52), acalabrutinib only (n = 10), or both ibrutinib and acalabrutinib (n = 6)
- Patient characteristics were also similar among these subgroups.
- Median tumor load was highest for patients with prior ibrutinib exposure, followed by those exposed to acalabrutinib.
- Ki-67 proliferation index ≥50% was reported in 66%, 60%, and 100% of these patients, respectively.
Median number of prior therapies was three in all subgroups.
Product characteristics were similar, in terms of transduction rate, CD4/CD8 ratio, CCR7+ T cells, CCR7- effector/effector memory T cells, and interferon gamma by co-culture, among all MCL morphology subgroups and prior BTKi subgroups.
High response rates were reported across subgroups by morphology and prior BTKi exposure (Figure 1).
Figure 1. Best response among subgroups*
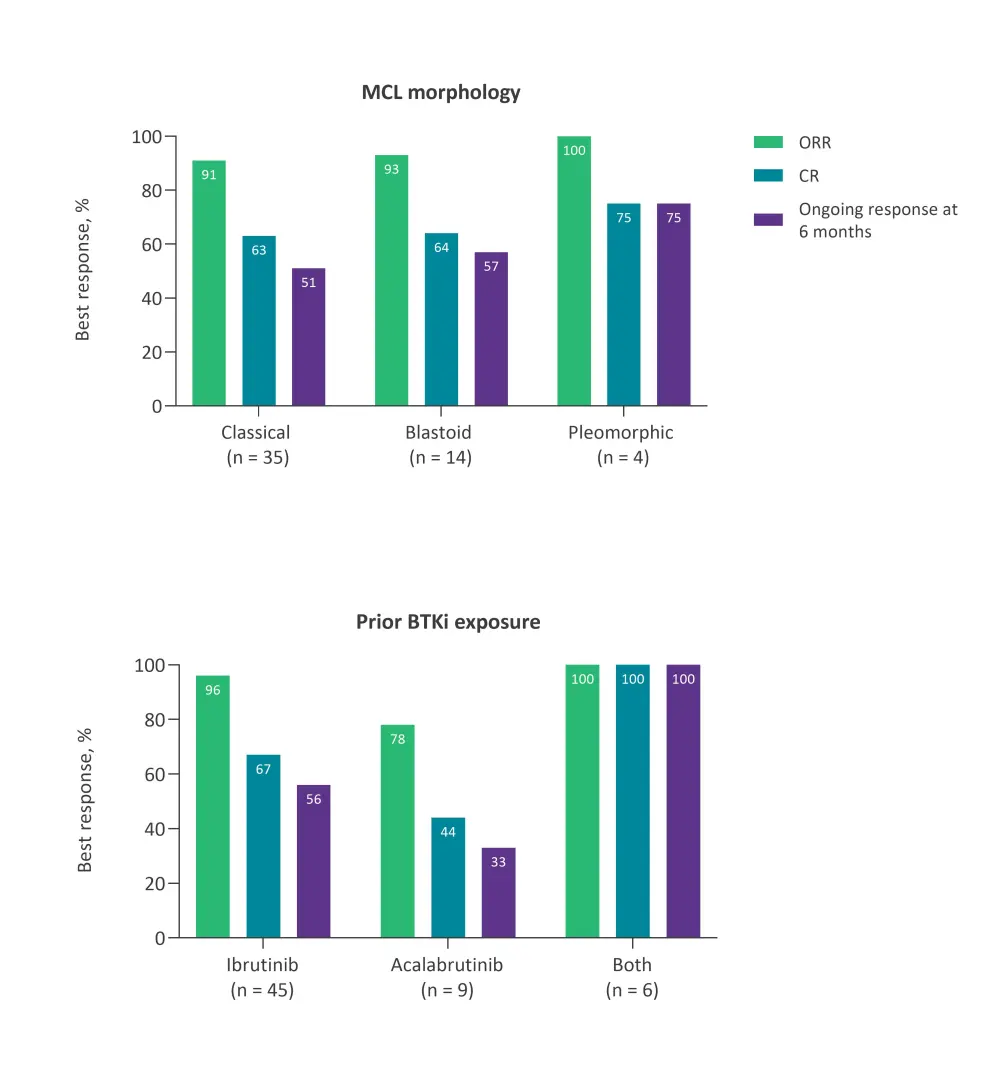
BTKi, Bruton’s tyrosine kinase inhibitor; CR, complete response; MCL, mantle cell lymphoma; ORR, overall response rate.
*Adapted from Wang et al.1
The 12-month OS rates were as follows:
- MCL morphology subgroups
-
- Classical: 85.7% (95% CI, 69.0–93.8)
- Blastoid: 71.4% (95% CI, 40.6–88.2)
- Pleomorphic: 100.0% (95% CI, not estimable [NE]–NE)
- Prior BTKi exposure subgroups
-
- Ibrutinib: 82.0% (95% CI, 67.2–90.6)
- Acalabrutinib: 77.8% (95% CI, 36.5–93.9)
- Both: 100% (95% CI, NE–NE)
Cytokine release syndrome (CRS) and neurologic events were also comparable across subgroups. Grade ≥3 events are provided in Table 1.
Table 1. Grade ≥3 CRS and neurologic events by subgroups*
|
CRS, cytokine release syndrome; MCL, mantle cell lymphoma. |
||||||
|
Grade ≥3 events, % |
MCL morphology |
BTKi exposure |
||||
|---|---|---|---|---|---|---|
|
Classical |
Blastoid |
Pleomorphic |
Ibrutinib |
Acalabrutinib |
Both |
|
|
CRS |
15 |
6 |
25 |
17 |
10 |
0 |
|
Neurologic events |
38 |
18 |
50 |
31 |
10 |
67 |
In terms of pharmacodynamic and pharmacological characteristics,
- The levels of proinflammatory cytokines were different among MCL morphology subgroups, and chemokine levels were more highly correlated for classical and pleomorphic MCL.
- Peak levels of proinflammatory cytokines, chemokines and CAR T cells were generally higher for patients with prior ibrutinib exposure compared with prior acalabrutinib exposure.
- The translational observations confirmed toxicity findings across prior BTKi subgroups.
Overall, KTE-X19 was associated with high response rates across two subgroup sets. Lower rates of Grade ≥3 CRS and neurologic events were reported in patients who had not received prior ibrutinib. Prior ibrutinib exposure appeared to demonstrate increased CAR T-cell expansion and circulating inflammatory cytokines and chemokines, while acalabrutinib showed decreased CAR T-cell expansion and T1-related cytokines and chemokines.
The Lymphoma Hub recently spoke to Michael Wang about the impact of prior BTKi exposure on the efficacy of KTE-X19 in patients with MCL; this interview can be found here.
Long-term outcomes based on ≥1-year follow-up data
Efficacy and safety outcomes as of December 31, 2019, with a median follow-up of 17.5 months, were presented during the EBMT meeting. Primary analysis results (data cutoff was July 24, 2019) showed an overall response rate (ORR) of 93% (complete response [CR] rate, 67%) after a median follow-up of 12.3 months.
Efficacy outcomes were reported from 60 evaluable patients (Figure 2).
At a median follow-up of 17.5 months (range, 12.3–37.6),
- Twenty-nine (48%) patients remained in response.
- Among patients achieving CR (n = 40), 70% remained in response.
Among those who were followed for a median of 32.3 months (range, 30.6–37.6), 39% stayed in remission without requiring any further therapy. In the entire study population (n = 74), ORR was 84%, with a CR rate of 59%.
Figure 2. Response in evaluable patients (N = 60)*
Figure 2. Response in evaluable patients (N = 60)*
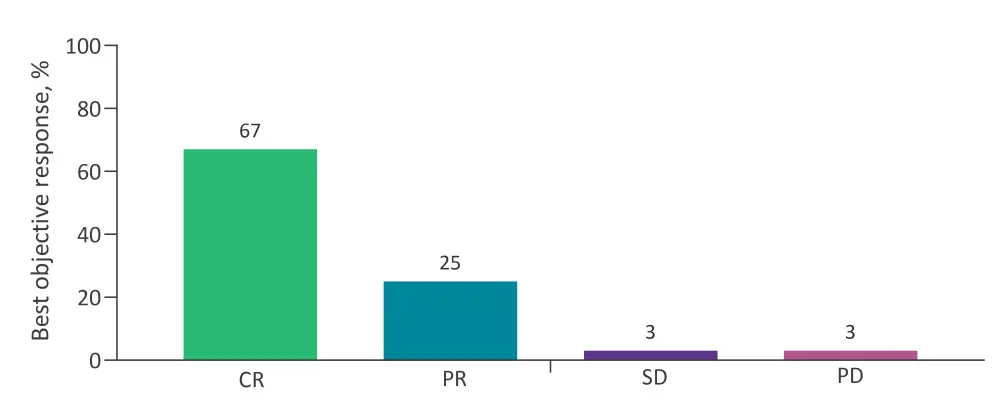
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
*Adapted from Wang et al.2
- Duration of response (DoR), progression-free survival (PFS), and OS were not reached.
- Adverse prognostic factors, including Ki-67 proliferation index, simplified Mantle Cell Lymphoma International Prognostic Index (s-MPIP), and TP53 mutation, had no impact on the rate of ongoing response.
Safety analysis indicated that there were no new safety signals during the additional follow-up, and no new CRS or Grade 5 events occurred since the last analysis. Any grade and Grade ≥3 adverse events (AEs), including anemia, neutropenia, and thrombocytopenia, decreased over time.
Between the two cutoff dates,
- 13% of patients experienced any Grade ≥3 AEs.
- The most common Grade ≥3 AE was neutropenia (9%), followed by thrombocytopenia (3%).
Higher CAR T-cell expansion was associated with achieving and maintaining a response. The association between pharmacokinetics and response/durability is currently being investigated. In patients with durable responses at 12 months, recovery of B cells increased and gene-marked CAR T cells decreased over time. There was no association between CAR T-cell expansion observed during the 2 weeks after infusion and B-cell aplasia.
In conclusion, KTE-X19 continues to demonstrate robust clinical activity and a manageable safety profile in patients with R/R MCL with a longer follow-up. Most patients with CR had durable responses at 12 months. Median DoR, PFS, and OS were not reached. Higher CAR T-cell expansion was observed in responders compared with non-responders, regardless of relapse at a later timepoint. Considering the groups earlier observation that most patients have detectable CD19 levels at relapse, this indicates that distinct mechanisms may cause primary and secondary treatment failure in this setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

