All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Bispecific antibodies for follicular lymphoma: frontline treatment and beyond
As previously reported on the Lymphoma Hub, bispecific antibodies such as mosunetuzumab and epcoritamab, which bind to CD20 on B cells and CD3 on T cells, remain promising regimens for the treatment of B-cell non-Hodgkin lymphoma (NHL).
Herein, we summarize recent data from three trials on mosunetuzumab and epcoritamab, presented during the 64th American Society of Hematology (ASH) Annual Meeting and Exposition. Bartlett presented the updated phase II results of mosunetuzumab monotherapy in patients with relapsed or refractory (R/R) follicular lymphoma (FL), initial results were published in the Lancet Oncology;1,2 while updated phase I/II results of subcutaneous epcoritamab plus rituximab and lenalidomide (R2) in patients with R/R FL, and initial phase I/II data on subcutaneous epcoritamab plus R2 in patients with previously untreated FL (both part of EPCORE NHL-2; NCT04663347) were presented by Falchi.3,4
Mosunetuzumab in R/R FL1
Mosunetuzumab is European Commission (EC) and recently Food and Drug Administration (FDA) approved for the treatment of R/R FL after two or more prior lines of therapy. The initial results by Budde et al.2 reported an ORR and CR of 80% and 60%, respectively, with durable responses seen across the majority of all-risk patients. Below, we report the longer median follow-up (28.3 months) study (GO29781; NCT02500407) results at the July 8, 2022, data cutoff.
Study design
This single-arm, multicenter phase II study included patients with Grade 1–3 FL, Eastern Cooperative Oncology Group (ECOG) Performance Status 0–1, and ≥2 prior therapies involving an anti-CD20 antibody and alkylator. Mosunetuzumab was given intravenously in 21-day cycles, with step-up dosing in Cycle 1.
Results
Overall, 90 patients enrolled with a median age of 60 years; 77% had advanced stage FL (stage III/IV), the median lines of prior therapy was three, 69% were refractory to last prior therapy, 79% were refractory to any prior anti-CD20 therapy, and 52% who had progression of disease within 24 months.
Efficacy
At follow-up an ORR and CR rate of 78% and 60%, respectively, was achieved; this is consistent with initial results.
Time to first response and time to first CR was 1.4 months and 3.0 months, respectively,
- median DOR and median duration of complete response (DOCR) was not reached;
- estimated DOCR at 12 and 24-month was 82% and 63%, respectively;
- the estimated median OS at 24-months was 84%;
- clinically meaningful ORR were seen across patients with common tumor mutation statuses in FL, including those with mutated TP53; and
- compared to last prior therapy, mosunetuzumab achieved higher response rates (ORR, 78% vs 56%; CR, 60% vs 36%), a longer DOCR, and improved PFS (24 vs 12 months, respectively).
Safety
Compared to initial data, no new Grade ≥3 or treatment-related adverse events (TEAEs) were reported. Overall, 51% of patients overall had a Grade 3-4 mosunetuzumab-related AE, mostly neutropenia and hypophostemia. The two deaths reported were deemed unrelated to mosunetuzumab, one due to progressive disease and the other unexplained. Four patients discontinued treatment due to AEs (cytokine release syndrome [CRS] in two, Hodgkin’s lymphoma in one, and Epstein Barr viremia in one). CRS events were mostly Grade 1 or 2 and occurred in Cycle 1; all were resolved. There was no difference in tumor response between those who had CRS vs without.
Epcoritamab in R/R FL3
Epcoritamab has demonstrated encouraging single-agent antitumor activity (EPCORE NHL-1; NCT03625037) and its combination with R2 led to high response rates and a manageable safety profile (arm 2a; EPCORE NHL-2) in patients with R/R FL. Below, we report Arm 2b of this study.
Study design
This is an open-label study which utilizes a less intense and more convenient dosing schedule compared to Arm 2a. The primary endpoints were safety and antitumor activity; inclusion criteria and treatment regimen are summarized in Figure 1.
Figure 1. Inclusion criteria and treatment regimen for Arm 2b of EPCORE NHL-2*
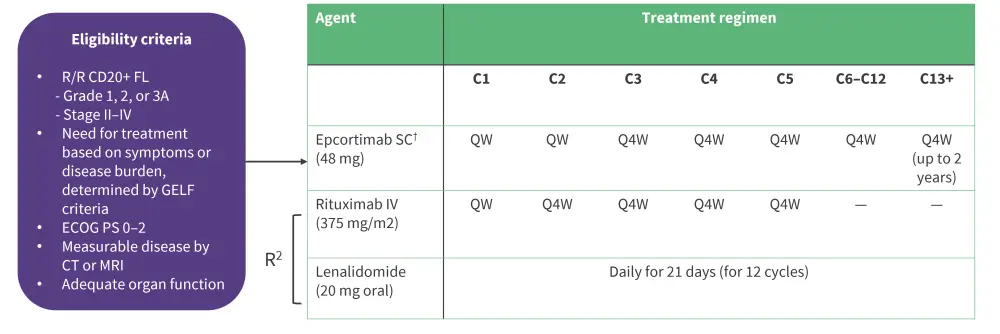
C, cycle; GELF, Groupe d'Etude des Lymphomes Folliculaires intravenous; QW, once weekly; Q4W, once every 4 weeks; R2, rituximab plus lenalidomide; SC, subcutaneous.
†In cycle one, patients were given step-up epcoritamab dosing and corticosteroid prophylaxis to mitigate cytokine release syndrome.
*Adapted from Falchi, et al.3
Results
At the first data cutoff on September 16, 2022, there were 76 patients in total with a median age of 64 years (range, 30–79 years); most (59%) had stage IV disease, 51% had high-risk disease defined as a follicular lymphoma international prognostic index score of 3–5, median time from prior therapy to first dose of epcoritamab and R2 was 16 months, and 30–40% had one or more of the following high-risk features: primary-refractory disease, double-refractory disease, progression of disease within 24 months, and refractory to last line of therapy.
Efficacy
At a median follow-up of 6.4 months, an ORR and complete metabolic response (CMR) rate of 95% and 80%, respectively, was achieved among evaluable patients (n = 66). Most CMRs were observed early (first tumor assessment was at 6-weeks), with some partial responses that converted to CMR during treatment. In total, 88% of patients remained in ongoing treatment. Deep responses were also observed across patients with high-risk features. A later data cutoff (October 31, 2022; median follow-up, 5.6 months), which included 79 patients, yielded higher ORR and CMR rates (96.2% and 83.5%, respectively) and the estimated PFS rate was 90% at the 6-month mark.
Safety
In the total cohort (N = 76), a median of six cycles of epcoritamab were given;
- 70% experienced a Grade ≥3 TEAE and 38% epcoritamab-related TEAE;
- three deaths were reported, all related to COVID-19;
- TEAE dose delays related to epcoritamab occurred in 25% of patients, with no discontinuations due to epcoritamab; and
- no clinical tumor lysis syndrome was observed and one patient experienced Grade 1 immune effector cell-associated neurotoxicity syndrome.
The most common TEAEs observed in ≥20% of patients were neutropenia, followed by CRS, and injection-site reaction (Figure 2); the latter two are likely to be directly related to epcoritamab. All CRS events were Grade 1–2 and resolved after a median of 2 days, with eight patients treated with tocilizumab and none resulting in treatment discontinuation. Most CRS cases occurred after the first full dose of epcoritamab.
Figure 2. Three most common TEAEs occurring in ≥20% patients*
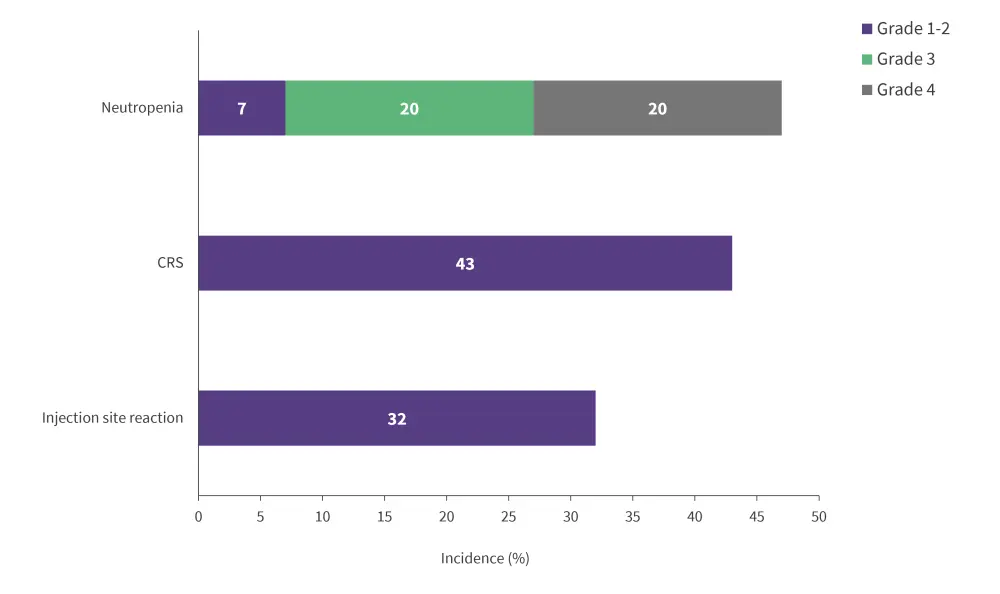
CRS, cytokine release syndrome; TEAE, treatment-emergent adverse event.
*Adapted from Falchi, et al.3
Epcoritamab in previously untreated FL4
Promising data on epcoritamab in R/R FL,3 led to its study within the first-line setting. Data from arm 6 of the EPCORE NHL-2 (NCT04663347) trial is reported below.
Study design
This open-label international trial included a total of 41 patients with previously untreated CD20+ FL of Grade 1-3A, with ECOG Performance Status 0–2, and a similar epcoritamab step-up dosing schedule as seen in Figure 1. Primary endpoints studied were antitumor activity and safety and the secondary endpoint was duration of response.
Results
In the total cohort, median age was 57 years; median time to first dose was 12 weeks; most patients (93%) had advanced stage disease, and 34% had a follicular lymphoma international prognostic index score of 3-5.
Efficacy
At the median follow-up of 8.1 months and data cutoff of September 16, 2022, ORR and CMR rates of 94% and 86% were achieved, respectively. Most responses were observed early (first assessment at 12 weeks), with some partial responders going on to achieve CMR during ongoing treatment. The median duration of response was not reached, with all patients, except one, maintaining responses at the 6-month mark.
Safety
Overall, 73% of patients experienced Grade ≥3 TEAEs, 34% of which were related to epcoritamab. Two deaths were reported (one due to COVID-19 and one due to septic shock) were unrelated to epcoritamab. TEAE dose delays and discontinuations related to epcoritamab occurred in seven and three patients, respectively. The most common AEs of any grade were CRS (54%) and neutropenia (47%), with other common AEs such as injection-site reaction (37%) likely related to epcoritamab. All CRS incidences were Grade 1-2 and resolved, with most occurring after the first full dose of epcoritamab.
Similar to the R/R FL study above, no clinical tumor lysis syndrome and only one case of Grade 1 immune effector cell-associated neurotoxicity syndrome was observed.
Conclusion
Data from these three studies show that mosunetuzumab monotherapy and epcortimab + R2 regimens are efficacious and well-tolerated in patients with R/R FL; this was also achieved with epcortimab + R2 in previously untreated patients with FL. Both studies on epcortimab + R2 reported high and deep responses at early assessment, with deep responses across high-risk R/R FL subgroups. The safety profile was consistent between the two arms with no high-grade CRS events reported. These data support both the further clinical investigation of epcortimab + R2 as a first-line therapy and the ongoing phase 3 EPCORE-FL-1 (NCT05409066) in R/R FL. Mosunetuzumab at additional follow-up achieved clinically meaningful responses in heavily pretreated patients with R/R FL and no new CRS events were reported; it also has the advantage of being an off-the-shelf fixed-duration therapy.
Given these data, mosunetuzumab and epcortimab + R2 represent promising treatment options for patients with follicular lymphoma.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




