All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
BTKi for patients with CLL: Class effects, guidelines, and real-world evidence
Do you know... According to current clinical and mechanistic evidence, which statement best reflects the role of off-target effects of BTK inhibitors in CLL?
The treatment landscape of chronic lymphocytic leukemia (CLL) has undergone significant change with the advent of targeted therapies, such as Bruton’s tyrosine kinase (BTK) inhibitors (BTKi).1 These BTKi, used as a monotherapy or in combination with other targeted therapies, such as B-cell lymphoma 2 (BCL-2) inhibitors (BCL-2i) and/or anti-CD20 monoclonal antibodies, have improved outcomes compared with the historical treatment option of chemoimmunotherapy.1,2
Class effects
BTKi are generally classified as covalent (irreversible) or non-covalent (reversible) based on their binding mechanism.2 Covalent BTKi include the first-generation BTKi ibrutinib and the second-generation BTKi acalabrutinib and zanubrutinib. Non-covalent BTKi include the third-generation BTKi pirtobrutinib.
B-cell receptor (BCR) signaling is a key driver of CLL pathogenesis, and BTK is a vital component of this pathway.3,4 In patients with CLL, BTKi can disrupt the BCR signaling cascade by binding to the BTK enzyme (Figure 1).3 Besides directly inhibiting BTK signaling, BTKi can also interact with several off-target kinases, such as epidermal growth factor receptor (EGFR), ErbB2, interleukin-2-inducible T cell kinase (ITK), and tyrosine kinase expressed in hepatocellular carcinoma (TEC).4 While these off-target effects likely contribute to the anti-CLL activity, they can also be associated with adverse events (AEs).4 In addition, BTKi can exert immunomodulatory effects within the CLL microenvironment, as BTK is expressed in non-leukemic cells.3,4 The Lymphoma Hub previously published an in-depth review of ibrutinib’s mechanism of action in CLL.
Figure 1. BTKi and BCL-2i mechanism of action in CLL*
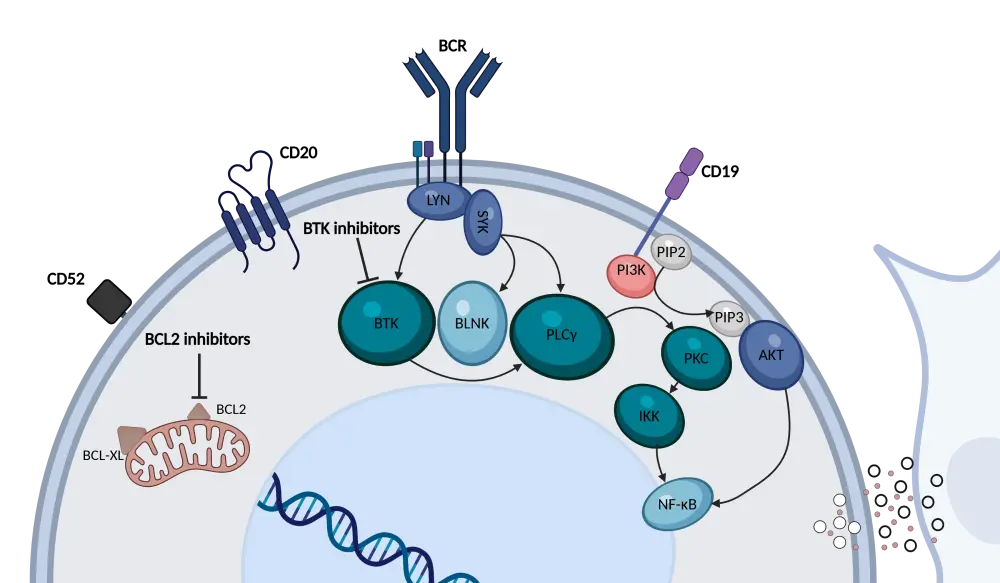
The safety profile of BTKi is closely linked to their kinase-binding patterns, with both on-target and off-target inhibition contributing to toxicities.1 The use of BTKi is associated with an increased risk of cardiovascular events, such as atrial fibrillation, hypertension, heart failure, sudden cardiac death, and ventricular arrhythmias.1 Bleeding events are common in patients treated with BTKi, although major events are rare.1 Hematologic toxicities, such as neutropenia and thrombocytopenia, are often reported following treatment with BTKi.1 Gastrointestinal events, dermatologic toxicities, arthralgias and myalgias, and headaches are also associated with BTKi treatment.1 Effective management of these AEs is essential to maintain treatment efficacy and patient quality of life. Optimal care involves supportive measures, dose adjustments, or switching therapies to avoid unnecessary discontinuation (Table 1).1
Table 1. Management recommendations for BTKi-associated selected AEs*
| AE | Management |
| Atrial fibrillation |
|
| Ventricular arrhythmia |
|
| Hypertension |
|
| Bleeding |
|
| Neutropenia |
|
| Thrombocytopenia |
|
| Diarrhea |
|
| Dermatologic toxicity and nail and hair changes |
|
| Arthralgias and myalgias |
|
| Headaches |
|
| *Adapted from Galitzia et al.1 AE, adverse event; BTKi, Bruton’s tyrosine kinase inhibitor; NSAID, non-steroidal anti-inflammatory drug. | |
Treatment guidelines
Both the National Comprehensive Cancer Network® (NCCN®) and the European Society for Medical Oncology (ESMO) guidelines incorporate BTKi monotherapy and combination therapies as treatment options for patients with treatment-naïve or relapsed/refractory (R/R) CLL (Figure 2).6,7
Figure 2. Key NCCN and ESMO guidelines for the treatment of A TN CLL and B R/R CLL*
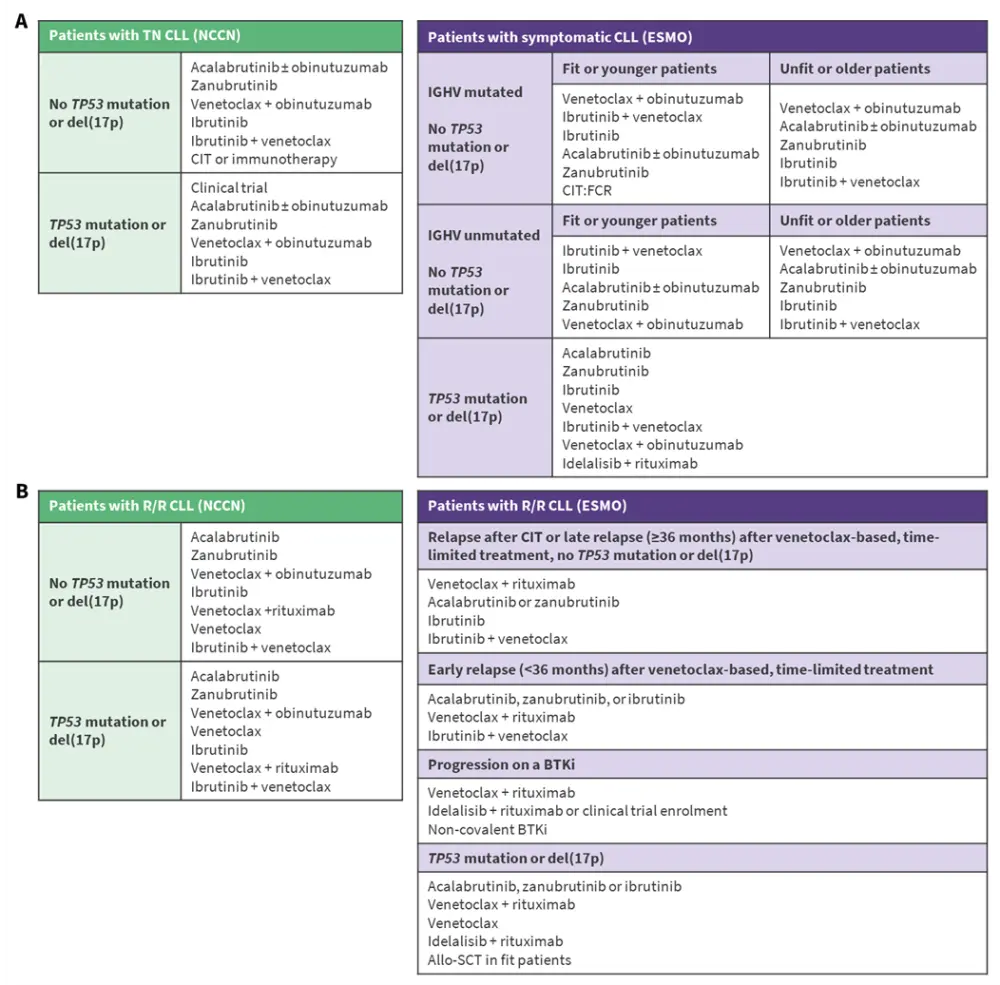
In first-line (1L) patients with CLL, treatment with BTKi can be used as continuous therapy, including ibrutinib, acalabrutinib ± obinutuzumab, or zanubrutinib, until progression, or as fixed-duration therapy with BTKi + venetoclax ± obinutuzumab.6,7 Molecular risk stratification and patient factors, such as age and comorbidities, should guide treatment decisions. For patients who relapse following chemoimmunotherapy or fixed-duration venetoclax-based therapy, continuous BTKi is an important treatment option.6 For patients who are resistant or intolerant to BTKi, switching to another BTKi can be considered.7 The non-covalent BTKi pirtobrutinib is an effective therapy for patients who are resistant/intolerant to covalent BTKi or for R/R patients following covalent BTKi-based therapy.6
The Lymphoma Hub previously spoke to Francesc Bosch, Vall d'Hebron Barcelona Hospital Campus, Barcelona, ES, who discussed factors influencing BTKi + BCL-2i treatment selection for patients with CLL.
What factors influence BTKi + BCL-2i treatment selection for patients with CLL?
Real-world studies
Real-world studies of BTKi in patients with CLL provide complementary evidence to randomized clinical trials, underscoring their sustained clinical activity and safety across treatment settings and patient subgroups. The Lymphoma Hub previously reported results from the real-world EVIdeNCE and REALITY studies of ibrutinib in patients with CLL.
Real-world survival outcomes in 1L ibrutinib-treated high-risk patients8
A large, retrospective study assessed real-world overall survival (rwOS) outcomes among patients with CLL treated with 1L single-agent ibrutinib with and without high-risk cytogenetic features using the Flatiron Health database from January 2011 to January 2023. This study included 1,242 patients, of whom 969 were high risk and 273 were not high risk. Patients were considered to have high-risk CLL if they had del(17p) (32.9%), del(11q) (36.7%), or unmutated IGHV genomic alterations (58.7%). The mean age was 70.0 and 70.8 years, and the median follow-up was 32 and 31 months in the high-risk and not high-risk groups, respectively.
The median duration of ibrutinib treatment was 18.9 and 14.3 months in the high-risk and not high-risk groups, respectively. The median rwOS was not reached for either group, with no significant difference in rwOS between the high-risk and not high-risk groups (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79–1.51; p = 0.60) (Table 2). In the sensitivity analysis using an alternative high-risk definition (presence of del(17p) or unmutated IGHV only) and in the subgroup of Medicare beneficiaries (high risk, 37.5%; not high risk, 39.6%), results were similar, with the median rwOS not reached in either group (Table 2).
Table 2. Cox proportional hazard model for rwOS among high-risk vs not high-risk patients with CLL treated with 1L ibrutinib*
| Analysis | HR (95% CI) | p value |
| Primary analysis | 1.09 (0.79–1.51) | 0.60 |
| Sensitivity analysis | 1.19 (0.86–1.64) | 0.30 |
| Medicare subgroup | 0.98 (0.63–1.53) | 0.94 |
| *Data from Allan JN, et al.8 1L, first-line; CI, confidence interval; CLL, chronic lymphocytic leukemia; HR, hazard ratio; rwOS, real-world overall survival. | ||
Results support the use of single-agent ibrutinib as 1L treatment for patients with CLL, irrespective of cytogenetic risk.
NAOS: Real-world efficacy and safety of acalabrutinib9
The retrospective, non-interventional, longitudinal NAOS study investigated the real-world use of acalabrutinib in 485 patients with CLL (1L, 58.8%; second-line+ [2L+], 41.2%) from 59 sites in France from 2021 to 2022. In total, 55.1% of patients had a prior cardiovascular history, 25.1% had del(17p)/mutated TP53, and 67.4% had unmutated IGHV. The median age was 73 and 77 years in the 1L and 2L+ groups, respectively.
The 12-month real-world progression-free survival (PFS) rate was 93.1% and 87% in the 1L and 2L+ groups, with no difference based on del(17p)/mutated TP53 (p = 0.21) and unmutated IGHV (p = 0.34) status. Treatment changes due to AEs occurred in 21.3% of patients, with Grade 3/4 AEs occurring in 9.6% and cardiac disorders reported in 2.1% of patients.
This large real-world study demonstrates that the efficacy and safety profile of acalabrutinib in a real-world setting is consistent with those reported in clinical trials.
Real-world efficacy and safety of zanubrutinib10
A multicenter, real-world study assessed the efficacy and safety of zanubrutinib monotherapy in 138 patients with CLL in China up until September 2024. In total, 65.2% of patients were treatment-naïve and 34.8% were R/R. The median age was 68 years. Of the 109 patients with available data, 24.8% had a primary TP53 deletion and/or mutation.
Among the 121 efficacy evaluable patients, the overall response rate and complete response rates were 86.0% and 8.3%, respectively. After a median follow-up of 36.8 months, the median PFS and overall survival (OS) were not reached. Patients with R/R CLL had a shorter PFS than treatment-naïve patients (p = 0.004), while OS was similar between the two groups (p = 0.069). The 36-month PFS rates were 77.7%, 84.3%, and 64.8% in the entire cohort, treatment-naïve patients, and R/R patients, respectively; the 36-month OS rates were 88.5%, 92.3%, and 83.0%, respectively. The presence of TP53 aberration did not impact PFS (p = 0.362) or OS (p = 0.512). In total, any-grade AEs and Grade ≥3 AEs occurred in 84.8% and 26.1% of patients, respectively.
Results from this study demonstrate comparable real-world efficacy and safety outcomes with zanubrutinib monotherapy, consistent with those observed in clinical trials.
REALITY-WW: Real-world 1L fixed-duration ibrutinib + venetoclax11
The REALITY-WW study is a prospective, observational study that aims to assess usage, factors that impact therapeutic decisions, and clinical response of 1L fixed-duration ibrutinib + venetoclax in patients with CLL in routine clinical practice. The primary endpoint is overall response rate, and key secondary endpoints include duration of response, PFS, OS, time to next treatment, tumor lysis syndrome risk, AEs, patient-reported outcomes, factors associated with physician decisions to initiate fixed-duration ibrutinib + venetoclax, and medical resource utilization. This study aims to enroll 200 patients across Europe, the Middle East, and Latin America.
The REALITY-WW protocol reflects the standard-of-care approach to treating patients with ibrutinib + venetoclax and allows physician and patient choice. The wide inclusion criteria will include patients who would not otherwise have been eligible for interventional clinical trials.
This educational resource is independently supported by Johnson & Johnson. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content





