All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the Lymphoma Coalition.
The lym Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the lym Hub cannot guarantee the accuracy of translated content. The lym and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Lymphoma & CLL Hub is an independent medical education platform, sponsored by AbbVie, BeOne Medicines, Johnson & Johnson, Miltenyi Biomedicine, Nurix Therapeutics, Roche, Sobi, and Thermo Fisher Scientific and supported through educational grants from Bristol Myers Squibb, Lilly, and Pfizer. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View lymphoma & CLL content recommended for you
Combination therapies for CLL: BTKi + BCL2i
Do you know... In the phase III GLOW clinical trial, how did ibrutinib + venetoclax impact independent review committee (IRC)-assessed complete response (CR) rates vs chlorambucil + obinutuzumab?
Several novel agents with distinct mechanisms of action (MoAs) have been developed recently for chronic lymphocytic leukemia (CLL).1,2 Inhibitors of Bruton’s tyrosine kinase (BTKi), B-cell lymphoma-2 (BCL2i), B-cell receptor signaling (BCRi), and phosphatidylinositol 3-kinase (PI3ki), have transformed both the treatment-naïve (TN) and relapsed/refractory (RR) treatment landscape.1,2 An overview of CLL, including current treatment strategies, and an in-depth review of ibrutinib’s MoA have been published previously on the Lymphoma Hub.
Here, we summarize the rationale for combining BTKi and BCL2i therapies, key clinical trial data supporting their role in the treatment of CLL, clinical management considerations, and future directions.
BTKi + BCL2i combinations: Rationale
Several phase III trials have confirmed the superior efficacy of BTKi (including ibrutinib, acalabrutinib, pirtobrutinib, and zanubrutinib) and the BCL2i venetoclax over chemoimmunotherapy (CIT) in both the TN and RR settings – including in patients with high-risk molecular features.1,3 CLL cell production, proliferation, survival, and migration are largely dependent on BCR and BCL2 pathway signaling, and BTKi and BCL2i are complementary in their MoA – allowing for a synergistic treatment combination.4 BTKi prevent BTK activation by hindering autophosphorylation and, thereby, suppressing downstream BCR signaling required for B-cell proliferation, migration, and survival.4 In addition, BTKi reduce cell surface expression of CXCR4, leading to rapid CLL cell redistribution from the spleen and lymph nodes to peripheral blood and, consequently, inducing transient lymphocytosis.4 BTKi also indirectly decrease MCL-1 protein expression, causing CLL cells to be highly dependent on BCL2 signaling.4 The addition of BCL2i that directly target BCL2 results in enhanced CLL cell apoptosis (Figure 1).4
Figure 1. BTKi mechanism of action and synergistic effects when combined with BCL2i*
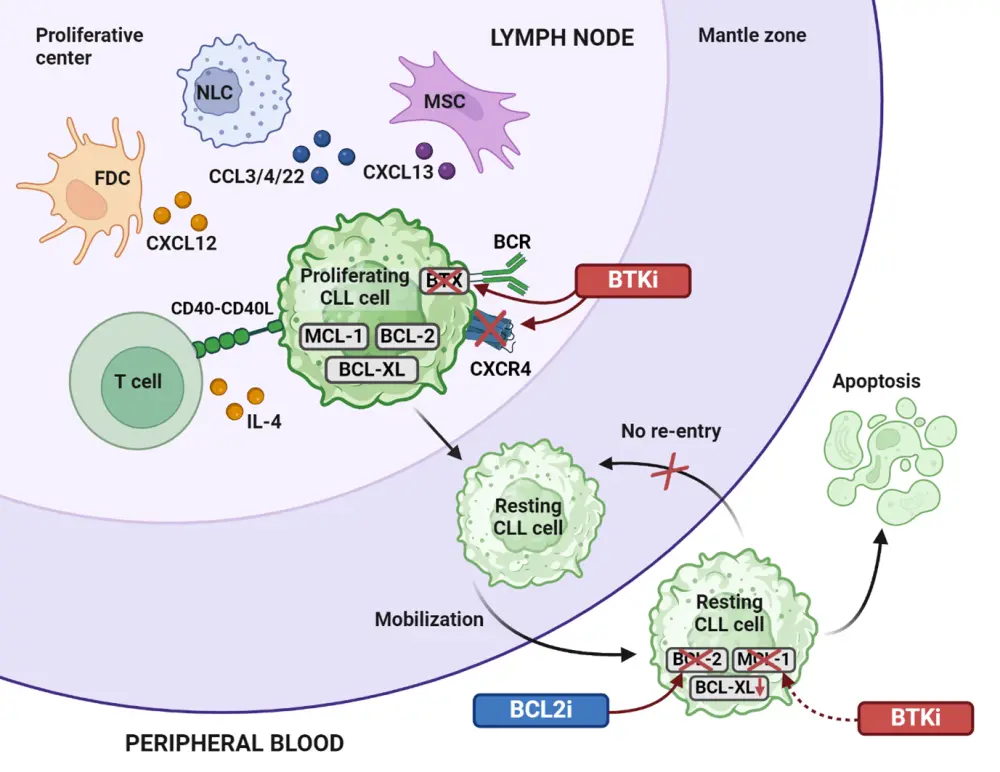
BCL2i, B-cell lymphoma-2 inhibitor; BCR, B-cell receptor; BTKi, Bruton’s tyrosine kinase inhibitor; CLL, chronic lymphocytic leukemia; CCL3/4/22, C-C motif chemokine ligands 3, 4, and 22; CXCL12/13, chemokine C-X-C motif ligands 12 and 13; CXCR4, C-X-C chemokine receptor type; FDC, follicular dendric cell; IL, interleukin; MSC, mesenchymal stem cell; NLC, nurse-like cell.
*Adapted from Timofeeva, et al.4 Created with BioRender.com.
BTKi + BCL2i combinations: Key trials
The European Commission (EC) granted approval to ibrutinib in combination with venetoclax as an oral fixed-duration regimen for the treatment of adult patients with previously untreated CLL in August 2022. The approval was based on key data obtained from the phase II CAPTIVATE (NCT02910583) and phase III GLOW (NCT03462719) trials.
CAPTIVATE
The CAPTIVATE trial showed that fixed-duration treatment with ibrutinib + venetoclax led to deep and durable responses in patients ≤70 years of age with previously untreated CLL.5 The CR rate was 55%, with 24-month progression-free survival (PFS) and overall survival (OS) rates of 95% and 98%, respectively.5
GLOW
The GLOW trial reported a superior PFS for ibrutinib + venetoclax over chlorambucil + obinutuzumab in elderly patients with CLL and/or those with comorbidities (Table 1).1,6 Patients receiving ibrutinib + venetoclax achieved significantly higher complete response (CR) and duration of response (DoR) rates than those treated with chlorambucil + obinutuzumab, with overall response rates (ORR) similar between treatment arms.1,6 The 4-year follow-up data of the GLOW trial demonstrated that fixed-duration ibrutinib + venetoclax continues to significantly improve PFS and achieve an OS advantage compared with chlorambucil + obinutuzumab in patients with previously untreated CLL.7
Table 1. Key efficacy data from the GLOW trial*
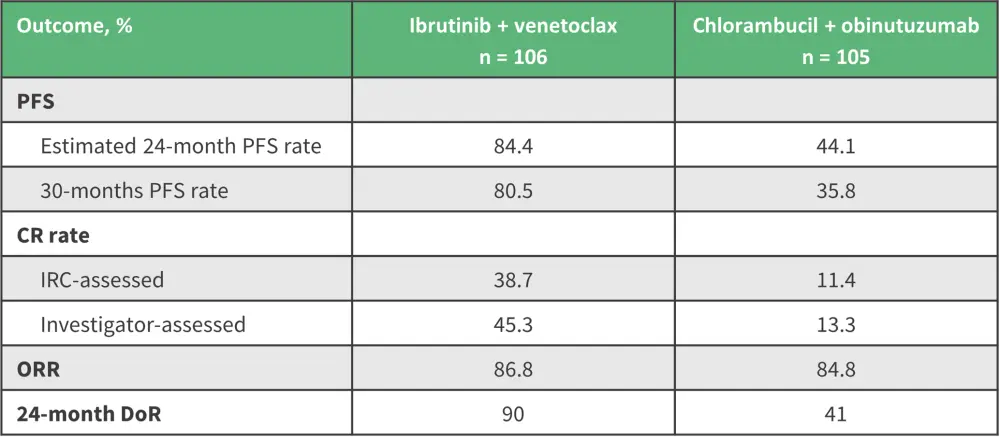
CR, complete response; DoR, duration of response; IRC, independent review committee; ORR, overall response; PFS, progression-free survival.
*Data from Kater, et al.6
For more details, see a discussion on the GLOW trial chaired by Astrid Pavlovsky during a recent Lymphoma Hub Steering Committee meeting.
Phase III GLOW trial: Ibrutinib plus venetoclax in previously untreated CLL/SLL
CLARITY
The phase II CLARITY trial (ISRCTN13751862) demonstrated the efficacy and safety of ibrutinib + venetoclax in the RR setting, with an ORR of 89% and 51% of patients achieving a CR or CR with incomplete BM recovery.8 Early measurable residual disease (MRD) negativity resulted in sustained MRD and clinical responses.9
SEQUOIA
Preliminary results from Arm D of the phase III SEQUOIA trial (NCT03336333), in which patients with high-risk TN CLL/SLL del(17p) and/or TP53 mutation received zanubrutinib + venetoclax combination treatment, demonstrated promising efficacy and tolerability, with a 100% ORR, a CR/stringent CR in 45% of patients, and a 24-month estimated PFS rate of 94%.10
CLL2-GIVe
Several studies have investigated the addition of agents with non-overlapping MoAs to BTKi + BCL2i combinations.1,3 Addition of the anti-CD20 monoclonal antibody obinutuzumab has been studied extensively, including in the phase II CLL2-GIVe trial (NCT02758665) which investigated ibrutinib + venetoclax + obinutuzumab in patients with TN CLL.1,3,11 Final analysis from the trial demonstrated that this triplet represents a highly active, time-limited treatment approach, with manageable tolerability.11 The 36-month OS and PFS rates were 92.6% and 79.9%, respectively, with high rates of MRD negativity achieved both during and at the end of treatment.11
Ongoing and recently completed phase III clinical trials for BTKi and BCL2i combinations in CLL are outlined in Table 2.
Table 2. Phase III clinical trials of BTKi and BCL2i combinations in treatment of CLL*
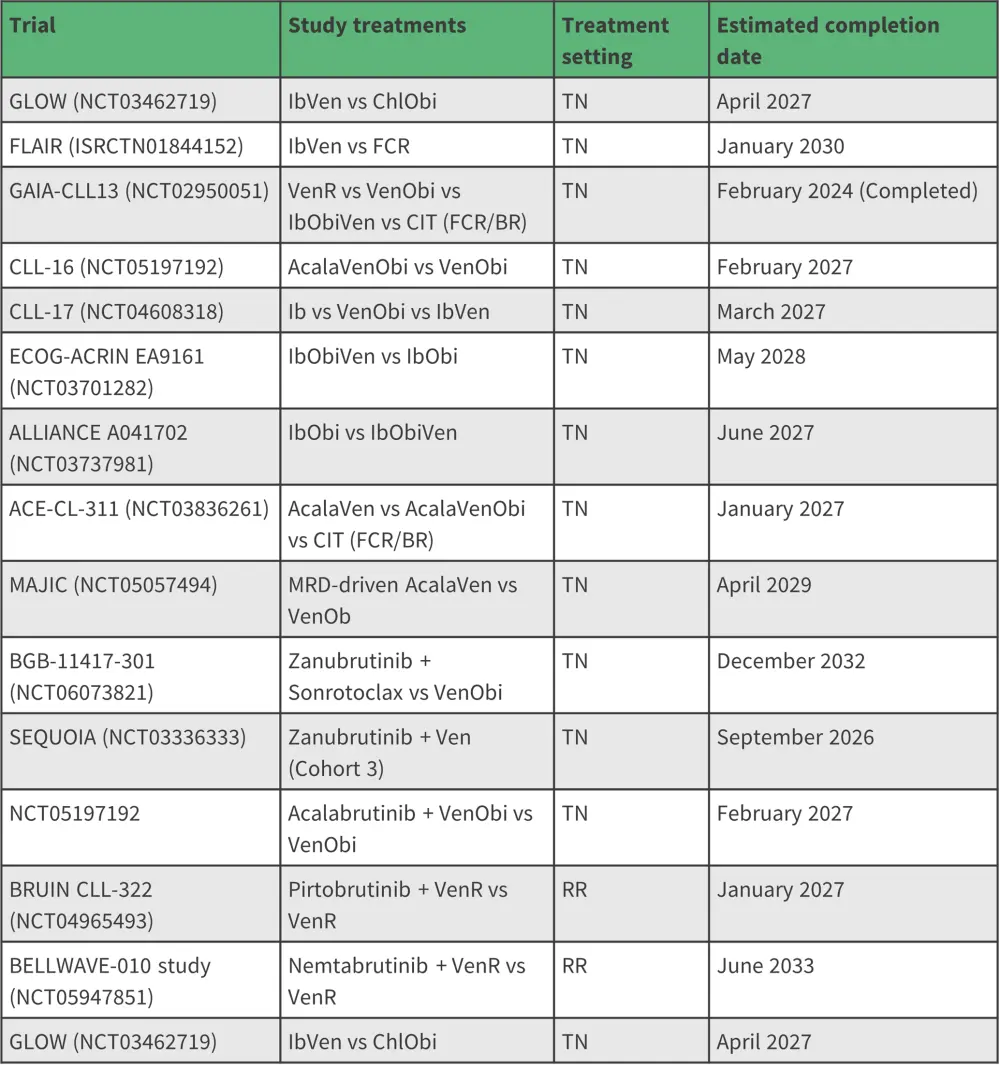
Acala, acalabrutinib; BR, bendamustine, rituximab; CIT, chemoimmunotherapy; FCR, fludarabine, cyclophosphamide, rituximab; Ib, ibrutinib; MRD, measurable residual disease; Obi, obinutuzumab; R, rituximab; RR, relapsed/refractory; TN, treatment-naïve; Ven, venetoclax.
*Adapted from Hayama M et al.1
Question 1 / 1
What was the 30-month PFS rate in the phase III GLOW trial with ibrutinib + venetoclax vs chlorambucil + obinutuzumab?
A
60.5% vs 25.8%
B
70.5% vs 38.5%
C
80.5% vs 35.8%
D
70.5% vs 35.8%
BTKi + BCL2i combinations: Clinical management considerations
Toxicity management
Ibrutinib has a well-defined long-term toxicity profile, with over 10 years of follow-up data available from the RESONATE-2 study.12–14 Adverse events (AEs) are the most likely cause of ibrutinib discontinuation and include heart arrhythmias, bleeding, diarrhea, arthralgias, hypertension, and infections.12 Atrial fibrillation should be managed using anticoagulants and rate/rhythm control, and hypertension managed with antihypertensives.12 For patients experiencing symptomatic ventricular arrhythmias, treatment hold or discontinuation of therapy with cardiological assessment to determine risk of recurrent cardiac events is recommended, although definitive discontinuation of BTKis may be necessary if the arrhythmia is life-threatening.12 Next-generation BTKi such as acalabrutinib and zanubrutinib are more selective, exhibiting less off-target activity, and may offer improved tolerability.12
The AE profile for patients with CLL treated with venetoclax includes both hematologic events such as neutropenia, and nonhematologic events such as tumor lysis syndrome (TLS) and gastrointestinal disorders.15 In cases of Grade 3 neutropenia with infection or fever, or Grade 4 neutropenia at first occurrence, interruption of venetoclax is recommended until neutropenia is reduced to Grade 1 or baseline level, with subsequent continuation of venetoclax administration at pre-interruption dose.15 For substantial neutropenia, growth factor support should be provided according to standards of care.15 TLS prophylaxis and monitoring should include tumor burden risk assessments, with hydration (oral/IV), antihyperuricemics, and rasburicase considered for hyperuricemia as per risk category and institutional guidelines.15 Antiemetics can be administered as needed for nausea and vomiting.15 The safety profile of ibrutinib + venetoclax has been reported as similar to profiles for each agent as monotherapy; including AEs of special interest such as atrial fibrillation.16
Continuous vs fixed-duration therapy
Combination treatments have enabled the potential for a movement from continuous BTKi therapy to the option for fixed-duration (FD) therapy, with MRD-directed strategies also under investigation. Benefits of FD vs continuous therapy include ease of administration and treatment breaks, which can impact on quality of life.17 Several studies have evaluated time-limited BTKi + BCL2i combinations, with or without the addition of obinutuzumab; with both FD- and MRD-adapted treatment-duration approaches investigated.1,3 Remissions appear to deepen in a proportion of patients during a second year of FD ibrutinib + venetoclax therapy compared with FD therapy of 1 year.3 This approach has generally demonstrated good tolerability, with high rates of MRD negativity and durable off-treatment remissions in a high proportion of patients.3 Time-limited ibrutinib + venetoclax is preferred to continuous BTKis, due to reduced toxicity and the possibility of retreatment at relapse.18 Long treatment-free intervals have been observed with FD therapy, especially in patients with CLL and favorable risk profiles.18 Studies suggest that patients prefer FD oral therapy over treatment-to-progression regimens.17 The ESMO clinical practice guidelines recommend that tolerability and reimbursement for prolonged treatment be considered when deciding between FD ibrutinib or personalized treatment based on MRD.18
Personalizing treatment
Various BTKi + CD20 and BCl2i + CD20 combinations are approved for the treatment of CLL.18,19 In a recent interim update to the ESMO Clinical Practice Guideline on new targeted therapies for TN and RR CLL, the BTKi + BCL2i combination was added to the recommendations, with ibrutinib + venetoclax recommended across for the treatment of several patient subgroups in both TN and RR settings (Figure 2).1,18,19 While the combination is not FDA approved, the NCCN guidelines recommend its use in both first and second line therapy.19
Figure 2. Key NCCN and ESMO guidelines for the treatment of A TN CLL and B RR CLL*
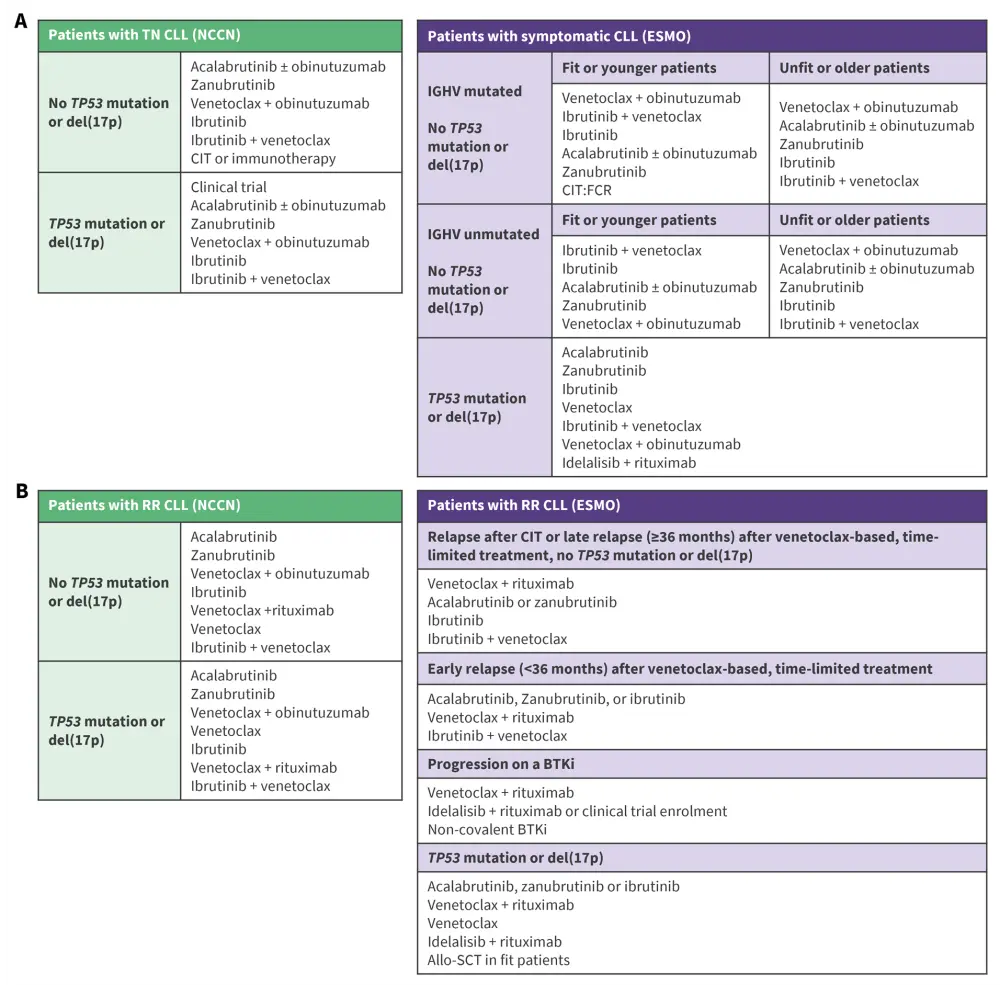
*Adapted from Eichhorst B et al.18 and NCCN.19
allo-SCT, allogeneic stem cell transplantation; BTKi, Bruton’s tyrosine kinase inhibitor; CIT, chemoimmunotherapy; CLL, chronic lymphocytic leukemia; del, deletion; ESMO, European Society for Medical Oncology; FCR, fludarabine, cyclophosphamide, rituximab; IGHV, immunoglobulin heavy chain variable; mAb, monoclonal antibody; NCCN, National Comprehensive Cancer Network; RR, relapsed/refractory; TN, treatment-naïve.
Choice of recommended therapy should include pre-treatment evaluation of IGHV and TP53 status, deletions in chromosome 17p [del(17p)] and/or TP53 mutations, and consideration of patient-related factors including co-medication, comorbidities (especially cardiac assessment if BTKi use is intended; ibrutinib + venetoclax should be considered carefully in older patients with cardiac comorbidities), patient preferences, availability of therapies, and treatment adherence expectations.18
Clinicians may have doubts about combining two key efficacious drug classes (BTKi and venetoclax) for the treatment of CLL in the first-line setting, with concerns about effective salvage therapies at progression.3 Although more data are required, current data suggest that very few patients develop resistance to BTKi/venetoclax during or following time-limited combination therapy.5 Emerging data from the CAPTIVATE FD cohort have demonstrated almost universal responses to ibrutinib monotherapy retreatment following progression with BTKi + BCL2i therapy.3,20 A retrospective study of ibrutinib + venetoclax in ibrutinib-resistant or double-refractory patients concluded that the combination can serve to fill an unmet need in options for patients with disease refractory to several common novel agents.21 Data on re-treatment with a BTKi + BCL2 combination are currently limited, and whether combination therapies are superior to sequential monotherapy remains to be determined.3
BTKi + BCL2i combinations: Conclusions and future directions
Several studies have demonstrated the benefits of the synergistic BTKi + BCL2i treatment combination, which offers a promising option for CLL patients across TN and RR settings. BTKi + BCL2i combinations provide efficacious and well-tolerated FD treatment options, with or without obinutuzumab.3,16 The combination has been approved in Europe and is recommended in ESMO guidelines. Upcoming data from ongoing trials may further support the use of these regimens and impact upon clinical guidelines. Personalized approaches with novel agents and combinations based on a comprehensive understanding of resistance mechanisms have the potential to improve patient outcomes and overcome clinical challenges subsequent to BTKi and BCL2i therapy.1,2
This educational resource is independently supported by Pharmacyclics LLC, an AbbVie Company, and Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
Your opinion matters
As a result of this content, I commit to reviewing the latest data on BTKi + BCL2i combinations to guide my treatment of CLL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content





